Resonance
The phenomenon of resonance was put given by Heisenberg to explain properties of the some of the molecules.
In case of the certain molecules, a single Lewis structure cannot explain all properties of molecule. The molecule is then assumed to have several structures, each of which can explain almost all the properties of the molecule but none can explain all properties of the molecule. The actual structure of it is in between of all these structures which are contributing and is known as resonance hybrid and the different individual structures are called as resonating structures or the canonical forms. This phenomenon is termed as resonance.
To Explain this, consider a molecule of the ozone O3 .
As the resonance hybrid of the above two structures (a) and (b). For simplicity, ozone can be represented by structure (c), which shows resonance hybrid having equal bonds between the single and double.
Resonance is generally shown by the structures of benzene, toluene, O3, allenes (>C = C = C<), CO, CO2, , SO3, NO, NO2 while it is not shown by the structures of H2O2, H2O, NH3, CH4, SiO2.
As a result of the resonance, the bond lengths of single and double bond in the molecule become equal for example O-O bond lengths in the molecule of ozone or C-O bond lengths in 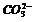 ion.
ion.
The resonance hybrid has the lower energy and therefore greater stability than any of contributing structures.
Greater is number of canonical forms especially with the nearly same energy, greater is stability of the molecule.
The difference among the energy of resonance hybrid and that of the most stable of resonating structures (possesing the least energy) is called resonance energy. Therefore,
The Resonance energy = Energy of resonance hybrid - Energy of the most stable of resonating structure.
In case of the molecules or ions having resonance, the bond order changes and which can be calculated as follows,
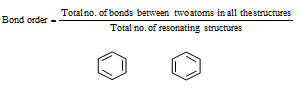
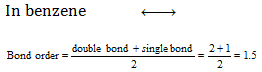
In Carbonate ion
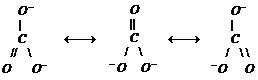
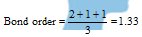
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Resonance questions? Resonance topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Resonance related problems. We provide step by step Resonance question's answers with 100% plagiarism free content. We prepare quality content and notes for Resonance topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours