Relative strength of acids and Bases
In practice Ka is used to define the strength only of those acids that are weaker than H3O+ and Kb is used to define the strength of only those bases that are weaker than OH-. For two weak acids HA1 and HA2 of ionisation constant Ka1 and Ka2 respectively at the same concentration then we can say that
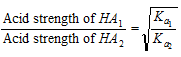
Similarly, relative strengths of any two weak bases at the same concentration are given by the ratio of the square-roots of their dissociation constants which means
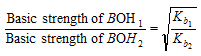
(1) The relative strength of the Inorganic acids
(i) Hydrides
(a) The acidic strength increases with increase in the electronegativity of the element directly attached with hydrogen.
H-F>H-OH>H-NH2>H-CH3>HCl>H2S>PH3>SiH4
(b) The acidic strength increases with increase in atomic size,
HF<HCl<HBr<HI;H2O<H2S<H2Se<H2Te
(ii) Oxyacids
(a) Among oxyacids of the same type formed by the different elements, acidic nature increases with the increasing electronegativity,
HOI < HOBr < HOCl; HIO4 < HBrO4 < HClO4
(b) In the oxyacids of same element, acidic nature increases with the increase in its oxidation number
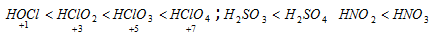
(c) The strength of the oxyacids increases from left to right across the period
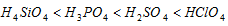
(d) For the identical oxidation state and configuration of the elements, acid strength decreases with the increase in size of atom.
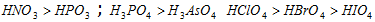
(2) Relative strength of organic acids
(i) The compound is said to be acidic in nature, if the conjugate base of it can stabilize through the process of resonance. Therefore phenol is acidic while ethanol is neutral because the conjugate base of phenol (C6H5O-) can be stabilized through the resonance while that of alcohol (C6H5O-) cannot.
(ii) Hydrogen atom attached to sp-hybridized carbon is more acidic than that on sp2 hybridized carbon which in turn is more acidic than that on sp3 hybridized carbon.
Thus, 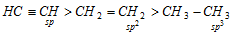
(3) The Relative strength of the Inorganic bases
(i) The basicity of a compound decreases with increase in electronegativity of the atom holding the electron pair, 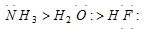
(ii) The larger size of the atom holding unshared electrons, the lesser will be the availability of electrons.
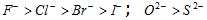
(iii) Presence of negative charge on the atom holding the electron pair increases basicity, while presence of positive charge on the atom holding the electron pair decreases the basicity. OH- > H2O > H3O+
(iv) Among the alkali and alkaline earth hydroxides (oxides) basic nature increases with the electropositivity
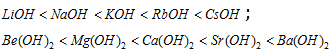
CsOH is the strongest known base
(v) On moving down the group; the basic nature decreases with the size of the central atom due to decrease in ability to donate the lone pair.
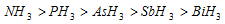
(4) Relative strength of Organic bases
(i) The higher electron density on nitrogen, more will be the basic character of amine.
(ii) A compound is basic in the nature, if the conjugate acid of it can be stabilized through the resonance. Hence guanidine 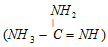 is as strong alkali as metal hydroxides because its conjugate acid
is as strong alkali as metal hydroxides because its conjugate acid 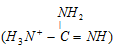 is very much stabilised through resonance.
is very much stabilised through resonance.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Relative strength of acids and Bases questions? Relative strength of acids and Bases topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Relative strength of acids and Bases related problems. We provide step by step Relative strength of acids and Bases question's answers with 100% plagiarism free content. We prepare quality content and notes for Relative strength of acids and Bases topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours