Reduction to free metal : Some of the methods commonly used to get free metal from the concentrated ore are given below,
(i) Smelting : The process of extracting a metal in the state of fusion is termed as smelting. In this method the ore is mixed with carbon, which is a resultent after the above reactions and heated in suitable furnace. A appropriate flux is added during the operation to convert the non-fusible gangue to fusible slag. Metallic oxide is reduced by carbon and the metal may be obtained in the molten state or as vapours which are condensed. The metals such as tin, lead or zinc are obtained by this process.
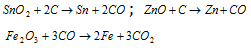
Flux and slag : Flux is a substance that is added during smelting to convert infusible silicons or earthy impurities into fusible material known as slag. Impurities + Flux = Slag. The slag is immiscible with the metal and has a low melting point and density. The slag floats over the metal and protects it from oxidation. It is seperated from the furnance through the slag hole. If the impurities present in the ore are acidic (SiO2) in nature, a basic flux such as MgO, FeO CaO, etc. are added; and if the impurities are basic (CaO, FeO, etc.) then on acidic flux (SiO2) is used. The gangue or matrix present in the ore is refractory or non-fusible in nature but it reacts with the flux forming fusible slag which does not mix with the molten metal and forms the upper layer. Slag are commonly silicates.
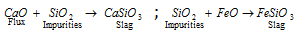
(ii) Reduction by Aluminium (Gold-schmidt alumino thermic process) : The process of reduction is used in the case of those oxides which cannot be easily reduced by carbon. In this procedure, metallic oxides ore are mixed with aluminium powder usually called as thermite and placed in the steel crucible lined inside with the refractory material and ignited by magnesium ribon. By this process a number of metals such as chromium and manganese are attained on a commercial scale in highly pure state.
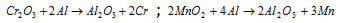
Huge amount of heat energy is released during reduction, which fuses the alumina and the metal both.
(iii) Self reduction process : This process is also called autoreduction process or air reduction process. The sulphide ores of the less electropositive metals such as Pb, Hg, Cu are heated in air as to convert part of the ore into oxide or sulphate which then reacts with left sulphide ore to give the metal and sulphur dioxide. No external reducing agent is taken in use in this process.
(iv) Electrolytic reduction process : This method is used in the extraction of alkali and alkaline earth metals, zinc and aluminium. The matter from which the metal to be attained is first smelted by the heating and then electrolysed. At times, some other salt is mixed to lower the melting point of substance taken. Example for it is as follows
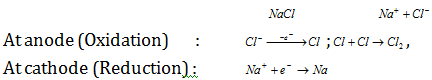
(v) Precipitation or metal displacement method (Hydrometallurgy) : This process is used for extraction of metals like cadmium, copper, gold and silver. A metal possesing higher electrode potential is added into the solution of a metal of the lower electrode potential with the result that the final is displaced or precipitated.
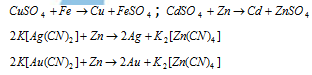
(vi) Amalgamation process: This method is used for the extraction of noble metals like silver, gold, etc., from native ores. The finely powdered ore is kept in contact with mercury which combines with particles of the metal present in the ore and forms the amalgam. The metal recovered from the amalgam by subjecting it to the distillation, where the mercury distills over the leaving behind the metal.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Reduction to free metal questions? Reduction to free metal topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Reduction to free metal related problems. We provide step by step Reduction to free metal question's answers with 100% plagiarism free content. We prepare quality content and notes for Reduction to free metal topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours