Reduction : Reduction is just reverse of oxidation. It is a process which involves; addition of hydrogen, removal of oxygen, removal of non-metal, addition of metal, decrease in +ve valency, gain of electrons and decrease in oxidation number.
(i) Removal of oxygen : 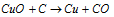
(ii) Addition of hydrogen : 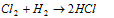
(iii) Removal of non-metal
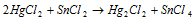
(iv) Addition of metal : 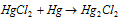
(v) Decrease in +ve valency
(a) 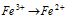 (+ve valency decreases)
(+ve valency decreases)
(b) 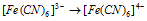 (-ve valency increases)
(-ve valency increases)
(vi) The gain of electrons (also known as electronation)
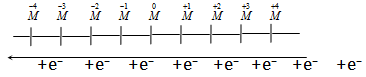
The gain of electrons
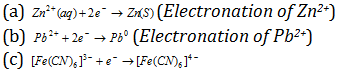 (Electronation of [Fe(CN)6]3-)
(Electronation of [Fe(CN)6]3-)
(vii) Decrease in oxidation number
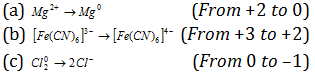
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Reduction Reactions questions? Reduction Reactions topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Reduction Reactions related problems. We provide step by step Reduction Reactions question's answers with 100% plagiarism free content. We prepare quality content and notes for Reduction Reactions topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours