The Real gases are those which does not obeys the Boyle's Charle's and gas law. To explain the behavior of real gases following two corrections are applied in the gas equation PV = RT
Volume correction: According to kinetic theory of ideal gases the volumes of molecule is presumed to be negligible where as the molecule occupy some volume due to their finite size (10-10m) consequently the whole volume of the container is not available for free kinetic motion of gas molecule as some fraction of it is occupied by the molecules themselves. Hence the effective volume of gas is = (V - b)
Pressure correction: According to ideal gas theory, inter molecular forces among the molecules is zero, But at high pressure and low temperature inter molecular distances decreases and consequently the intermolecular attractive forces converts into effective. As a result of this the molecules do not exert as much force as they could exert in the absence of attractive force. Hence the effective pressure would be greater than the observed pressure (P).
Hence the perfect pressure of gas is = (P+a/V2)
Equation for real gas: (Vander waal's equation)
(P+a/V2)(V-b = RT) for 1 mol. of Gas; (P+an2/V2)(V-nb = nRT) for n numbers of particle of Gas
Unit: α→ Newton × m4 ; b → m3 ;
Dimension: [a] = [ML5T-2] ; [b] = [L3]
The value of constants 'a' and 'b' relays upon the nature of gas
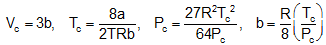
or 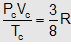 , Here Po = critical pressure, Tc = critical temperature, Vc = critical volume.
, Here Po = critical pressure, Tc = critical temperature, Vc = critical volume.
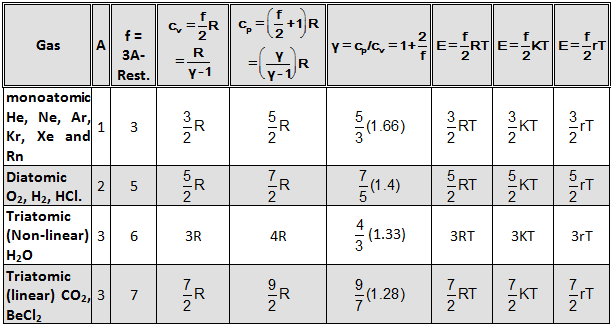
Various information in Table
Email based Physics assignment help - homework help at Expertsmind
Are you searching physics expert for help with Real Gas questions? Real Gas topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Physics assignment help and physics homework help. Live tutors are available for 24x7 hours helping students in their Real Gas related problems. We provide step by step Real Gas question's answers with 100% plagiarism free content. We prepare quality content and notes for Real Gas topic under physics theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving physics queries in excels and word format.
- Best tutoring assistance 24x7 hours