Quantum numbers
Each of the orbital in an atom is specified by the set of the three quantum numbers (which are n, l, m) and each electron is designated by the set of the four quantum numbers (which are n, l, m and s).
(1) Principle quantum number (n)
(i) This was proposed by Bohr and signified by 'n'.
(ii) This determines the average distance between the electron and nucleus, means it denotes the size of an atom.
(iii) This determines the energy of an electron in an orbit where electron is present.
(iv) The maximum number of the electron in an orbit represented by this quantum number as 2n2 No energy shell in the atoms of known elements contains more than 32 electrons.
(v) It provides the information of orbit K, L, M, N------------.
(vi) The angular momentum can also be calculated by using principle the quantum number
(2) Azimuthal quantum number (l)
(i) The azimuthal quantum number is also called as angular quantum number. Proposed by the Sommerfield and is denoted by 'l'.
(ii) This determines the number of sub shells or the sublevels to which the electron belongs.
(iii) This tells about the shape of the subshells.
(iv) This also expresses the energies of the subshells s < p < d < f (hear increasing energy).
(v) The value of l = (n-1) always. Where 'n' is the number of principle shell.
|
(vi) Value of l
|
=
|
0
|
1
|
2
|
3.....(n-1)
|
|
Name of subshell
|
=
|
s
|
p
|
d
|
f
|
|
Shape of subshell
|
=
|
Spherical
|
Dumbbell
|
Double dumbbell
|
Complex
|
(vii) This represents the orbital angular momentum. The angular momentum is equal to 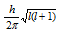
(viii) The maximum number of the electrons in the subshell = 2(2l + 1)

(ix) For the given value of 'n' the total values of 'l' is always equal to the value of 'n'.
(3) Magnetic quantum number (m)
(i) This was proposed by Zeeman and is denoted by 'm'.
(ii) This gives the number of permitted orientation of the subshells.
(iii) The value of m varies from -l to +l through the zero.
(iv) This tells about the splitting of the spectral lines in the magnetic field that is this quantum number proves the Zeeman effect.
(v) For the given value of 'n' the total value of 'm' is equal to n2
(vi) For the given value of 'l' the total value of 'm' is equal to (2l+1)
(vii) Degenerate orbitals : The orbitals having the same energy are called as degenerate orbitals. For example for p subshell px py pz
(viii) The number of the degenerate orbitals of s subshell =0.
(4) Spin quantum numbers (s)
(i) This was proposed by the scientist Goldshmidt & Ulen Back and is denoted by the symbol of 's'.
(ii) The value of 's' is +1/2 and -1/2, which denotes the spin or the rotation or direction of electron on its axis during the movement.
(iii) The spin might be clockwise or anticlockwise.
(iv) It represents the value of the spin angular momentum is equal to 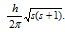
(v) The maximum spin of an atom = 1/2 * number of the unpaired electron.
(vi) This quantum number is not result of the solution of schrodinger equation as solved for the H-atom.
Table : Distribution of electrons among the quantum levels
|
n
|
l
|
m
|
Designation of orbitals
|
Number of Orbitals in the subshell
|
|
1
|
0
|
0
|
1s
|
1
|
|
2
|
0
|
0
|
2s
|
1
|
|
2
|
1
|
-1, 0, +1
|
2p
|
3
|
|
3
|
0
|
0
|
3s
|
1
|
|
3
|
1
|
-1, 0, +1
|
3p
|
3
|
|
3
|
2
|
-2, -1, 0, +1, +2
|
3d
|
5
|
|
4
|
0
|
0
|
4s
|
1
|
|
4
|
1
|
-1, 0, +1
|
4p
|
3
|
|
4
|
2
|
-2, -1, 0, +1, +2
|
4d
|
5
|
|
4
|
3
|
-3, -2, -1, 0, +1, +2, +3
|
4f
|
7
|
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Quantum numbers questions? Quantum numbers topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Quantum numbers related problems. We provide step by step Quantum numbers question's answers with 100% plagiarism free content. We prepare quality content and notes for Quantum numbers topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours