Quantitative analysis (Estimation of Elements) : After qualitative analysis of elements, the next step in the determination of molecular formula of an organic compound is the estimation of various elements by mass, i.e. finding the percentage composition of the elements by mass. The various methods commonly employed for the estimation of principal elements are discussed in the table.
Table: Quantitative estimation of elements in organic compounds
Element
|
Method and its principle
|
Formula
|
|
Carbon and Hydrogen
|
Liebig's combustion method : In this process, a given weight of organic compound is heated with pure and dry cupric oxide in a steam of pure and dry oxygen, when carbon is oxidised to carbon dioxide while hydrogen is oxidised to water. From the weight of CO2 and H2O, the percentage of C and H can be calculated.
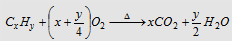
|

|
|
Nitrogen
|
(i) Duma's method : Elemental nitrogen is converted into molecular nitrogen by a suitable chemical method and its voiume is changed to STP data.
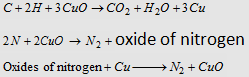
(ii) Kjeldahl's method : Nitrogen in organic compound is converted into NH3 by suitable chemical method which, in turn, is absorbed by V1 mL of N1H2SO4.
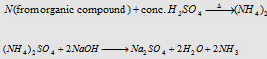
|
% of N = 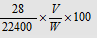
Where, V= volume of N2 in nitrometer (in ml) at NTP,
W= Weight of substance taken;
% of N = (1.4 * N * V)/ W
Note : This method is, however, not applicable to compounds containing nitrogen in the ring (e.g. Pyridine, quinoline etc) and compounds containing nitro and azo (- N = N -) groups since nitrogen in these compounds is not completely converted into (NH4)2SO4 during digestion.
|
|
Halogens
|
(i) Carius method : The method is based on the fact that when an organic compound containing halogen (Cl, Br, or I) is heated in a sealed tube with fuming nitric acid in presence of silver nitrate, silver halide is formed. From the mass of silver halide formed, the percentage of the halogen can be calculated.
|
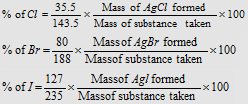
|
|
(ii) Schiff's and Piria method : In this method the accurately weighed organic compound (0.15 - 0.25 g) is taken in a small platinum crucible with a mixture of lime and sodium carbonate, (CaO + Na2CO3). It is now heated strongly and then cooled and dissolved in dilute nitric acid in a beaker. The solution is then filtered and the halide is precipitated with silver nitrate solution. Halogen is now calculated as in Carius method.
|
|
|
Sulphur
|
Carius method : When an organic compound containing sulphur is heated with fuming nitric acid, sulphur is oxidised to sulphuric acid. This is taken as barium sulphate by adding barium chloride solution. From the amount of barium sulphate, percentage of sulphur can be calculated.
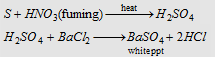
|
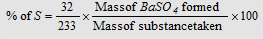
|
|
phosphorous
|
Carius method : The organic compound containing phosphorus is heated with fuming nitric acid. Phosphorus is oxidised to phosphoric acid. It is precipitated as magnesium ammonium phosphate, MgNH4PO4, by the adding up of magnesia mixture
(MgSO4 + NH4OH + NH4Cl). The magnesium ammonium phosphate is washed, dried and ignited when it is converted to magnesium pyrophosphate (Mg2P2O7).
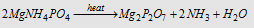
From the mass of magnesium pyro-phosphate, the percentage of phosphorus in the compound can be calculated.
|
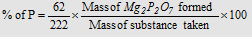
|
|
Oxygen
|
(i) The usual method of determining the percentage of oxygen in an organic compound is by the method of difference. All the elements except oxygen present in the organic compound are estimated and the total of their percentages subtracted from 100 to get the percentage of oxygen.
(ii) Aluise's method :. Organic compound containing oxygen is heated with graphite and CO formed is quantitatively converted into CO2 on reaction with I2O5.
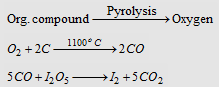
|
Percentage of oxygen = 100 - (Sum of the percentages of all other elements)
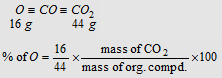
|
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Quantitative analysis of Organic Compounds questions? Quantitative analysis of Organic Compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Quantitative analysis of Organic Compounds related problems. We provide step by step Quantitative analysis of Organic Compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for Quantitative analysis of Organic Compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours