Wet tests for acid radicals : Salt or mixture is treated with dil. H2SO4 and also with conc. H2SO4 separately and by observing the types of gases evolved. Confirmatory checks of anions are done.
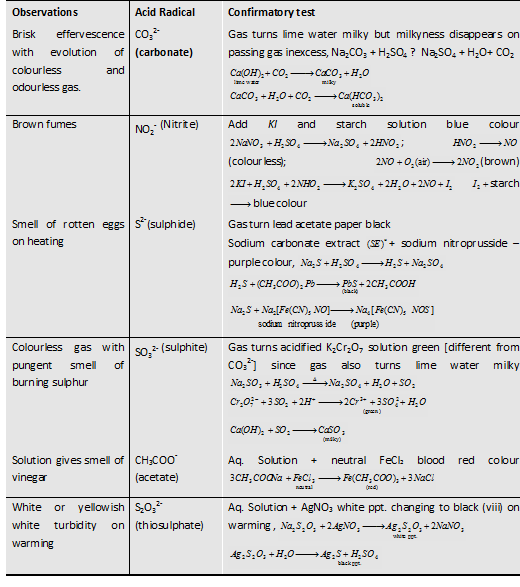
Table: Observations with Dilute H2SO4
|
Observation
|
Acid Radical
|
Confiramatory Test
|
|
Colourless pungent gas giving white fumes with aq. NH4OH
|
Cl- (chloride)
|
Add MnO2 in the same test tube and heat-pale green Cl2 gas (i)
S.E.+HNO3 + AgNO3 solution -white ppt. soluble in aq. NH3 (ii)
Chromyl chloride test (iii)
|
|
Reddish brown fumes
|
Br-(bromide)
|
Add MnO2 and heat -yellowish brown Br2 gas (iv)
S.E.+HNO3 + AgNO3 solution -pale yellow ppt. partially soluble aq. NH3 (v)
Layer test (vi)
|
|
Violet pungent vapours converting starch paper blue.
|
I-(iodide)
|
S.E.+HNO3 + AgNO3 → yellow ppt. insoluble in aq. NH3 (vii)
Layer test (vi)
|
Reactions
Chloride : (i) 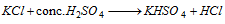
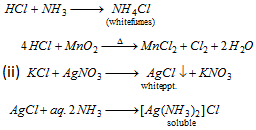
(iii) Chromyl- chloride test :
Chloride + 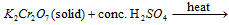 reddish brown vapours of chromyl-chloride. Pass these vapours into NaOH when yellow Na2CrO4 solution is formed. On adding CH3COOH and (CH3COO)2Pb, yellow ppt. of lead chromate is formed.
reddish brown vapours of chromyl-chloride. Pass these vapours into NaOH when yellow Na2CrO4 solution is formed. On adding CH3COOH and (CH3COO)2Pb, yellow ppt. of lead chromate is formed.
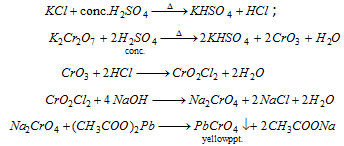
Bromide :
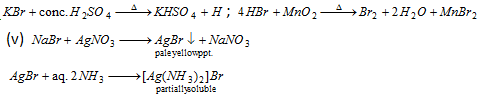 ;
;
(vi) Layer Test : S.E + Cl2 water +CHCl3 → yellowish orange colour in CHCl3 layer (CS2 or CCl4 can be taken instead of CHCl3);
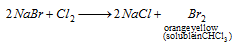
In case of I-, violet colour of I2 in CHCl3 layer, 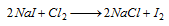 (violet)
(violet)
Iodide : (vii) 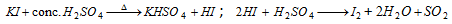
Nitrate : 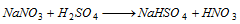
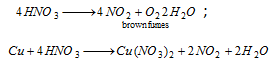
(viii) Ring test : To water extract (all NO-3 are water soluble) add freshly prepared FeSO4 solution and then conc. H2SO4 carefully by the side of the test- tube. A dark brown ring of [Fe(H2O)5NO]2+SO42-at the interface between the two liquids is formed.
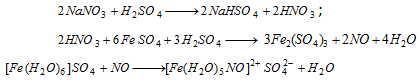
Oxalate : 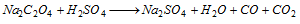
CO burns with blue flame and CO2 turns lime water milky.
(ix) 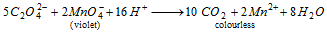
(x) 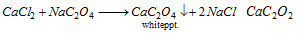 decolourises acidified KMnO4
decolourises acidified KMnO4
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Qualitative Analysis-Wet test for Acid Radicals questions? Qualitative Analysis-Wet test for Acid Radicals topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Qualitative Analysis-Wet test for Acid Radicals related problems. We provide step by step Qualitative Analysis-Wet test for Acid Radicals question's answers with 100% plagiarism free content. We prepare quality content and notes for Qualitative Analysis-Wet test for Acid Radicals topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours