Purification or refining of metals : Metals obtained from the above process are usually impure and require purification. Some of the methods used in refining of the metals are as follows,
(i) By poling : The molten metal's is stirred by green wood poles. Wood at high temperature of the molten metal's form hydrocarbons such as methane which being about the reduction of any oxide present in metal for example copper oxide present in the blister copper. In the case of the the impurities are oxidised and float on molten metal as the scum which is removed.
(ii) By cupellation : In this process the impure metal is heated in a blast of air when impurities are oxidised and blown away. For instance, when impure silver is heated in air, lead there in it is oxidised to litharge (PbO) and blown away leaving behind the shining of silver.
(iii) By liquation : This process is used for refining easily fusible metals like lead and tin. The metal which is not pure is heated on the slopy hearth of a reverberatory furnace. The metal melts and flows down leaving the impurities.
(iv) By distillation : Some of the metals posses very low melting point and soon vaporize on behind heating, while related impurities remains in solid state. Zinc, mercury and arsenic are purified by this process. Vacum distillation gives pure product and is used in the refining of metals of IA and IIA Groups.
(v) By fractional distillation : This method is applied for the separation of the cadmium from zinc. In metallurgy of zinc, the metal is invariably associated with the cadmium. The impure zinc is mixed with the powdered coke and heated when first portion of the condensate have cadmium while zinc is obtained in subsequent portions.
(vi) By thermal dissociation : In this method the metal is first converted into some compound which is decomposed into the pure metal by heating. For instance, nickel which is not pure is heated with the carbon monoxide at 60oC to form the nickel carbonyl Ni(CO)4 which is then decomposed at the higher temperature, 150-180oC to provide very pure nickel. At times iron is also purified by this method.
(vii) By Electrolytic refining : Most of the metals like nickel, silver, gold, zinc, copper, and chromium are purified electrolytically. The impure metal is made anode and a thin sheet of the pure metal as cathode in a suitable electrolytic bath. On passing current metal from the anode passes in the solution and the pure metal from the electrolyte is deposited on cathode. The electrolyte taken in use in the bath is usually a complex salt of metal to enable the smooth deposition of the pure metal on cathode.
(viii) Special processes
(a) Mond's process: Nickel is purified by this method. Nickel which is not pure is treated with carbon monoxide at 60-80°C when the volatile compound, nickel carbonyl, is produced. Nickel carbonyl decomposes at 180°C to form pure nickel and carbon monoxide which can again be used.
(b) Van Arkel process : This methods is generally applied for obtaining ultrapure metals. The metal which is not pure is converted into a volatile compound while the impurities are not affected. The volatile component is then decomposed electrically to obtain the pure metal. Ti, Zr, Hf, Si etc., have been refined by this method.
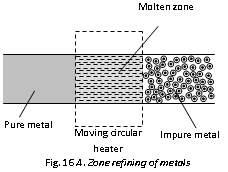
(c) Zone refining or the fractional crystallisation : Elements like Ga, Si, Ge, etc., are used as semiconductors are refined by this process. Highly pure metals are attained. The process is based on the difference in solubility of the impurities in molten and solid state of the metals. A movable heater is fitted around the rod of the metal. The heater is slowly moved across rod. The metal melts at point of heating and as heater moves on from one end of the rod to the other end, the pure metal crystallises while impurities pass on the adjacent melted zone.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Purification and refining of metal questions? Purification and refining of metal topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Purification and refining of metal related problems. We provide step by step Purification and refining of metal question's answers with 100% plagiarism free content. We prepare quality content and notes for Purification and refining of metal topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours