The Properties of solids
The properties of the solids which are useful in the electronic and magnetic devices such as, computers, transistor, and telephones etc., are summarized as follows,
(1) Electrical properties : Solids are classified into following classes depending on the extent of conducting nature.
(i) Conductors : The solids which allow the electric current to pass through them are known as conductors. These are further of the two types; Metallic conductors and electrolytic conductors. Electrical conductivity of these solids is high in the range 104 -106 ohm-1 cm-1. Their conductance decrease with the increase in temperature.
(ii) Insulators : The solids which do not allow the current to pass through them are called insulators. Such as wood rubber, and plastic etc. the electrical conductivity of this type of solids is very low that is
10-12 -10-22 ohm-1 cm-1.
(iii) Semiconductors : The semiconductors are the solids whose electrical conductivity lies between those of the conductors and insulators are known as semiconductors. The conductivity of the solid is because of the presence of the impurities. For instance Silicon and Germanium. Their conductance increase with the increase in temperature. The electrical conductivity of these solids is increased by adding impurity to them. This is termed as Doping. When silicon the is doped with P (or As, group 5th elements), we obtain n-type semiconductor. This is because of the reason that P has five valence electrons. It forms the four covalent bonds with silicon and the fifth electron remains free and is bound loosely. This give rise to the n-type semiconductor because the current is carried by electrons when the silicon is doped with Ga (or in ln/Al, group third elements) we obtain p-type semiconductors.
Superconductivity : once any material loses its resistance for the electric current, then it is known as superconductor, Kammerlingh Onnes (1913) observed this phenomenon at the temperature of 4K in mercury. The materials which are offering no resistance to the flow of the current at very low temperature (2-5K) are known as superconducting materials and phenomenon is termed as superconductivity.
Examples, Nb3, Ge alloy (Before 1986)
La1.25Ba0.15CuO4 (1986)
YBa2, Cu3O7 (1987)
The below written are the important applications of the superconductivity,
(a) Electronics,
(b) Building supermagnets,
(c) Aviation transportation,
(d) Power transmission
The temperature at which the material enters superconducting state is called as superconducting transition temperature, (Tc). The Superconductivity was also observed in the lead (Pb) at the temperature 7.2 K and in tin (Sn) at 3.7K. The phenomenon of the superconductivity in other materials like polymers and organic crystals. Examples of it are
(SN)x, polythiazyl, the subscript x indicates the large number of the variable size.
(TMTSF)2PF6, where the TMTSF is the tetra methyl tetra selena fulvalene.
(2) Magnetic properties : These are based on the behavior of substances when placed in magnetic field, there are generally classified into five classes.
Table : Magnetic properties of solids
|
Properties
|
Description
|
Alignment of Magnetic Dipoles
|
Examples
|
Applications
|
|
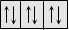
Diamagnetic
|
Feebly repelled by the magnetic fields. Non-metallic elements (excepts O2, S) inert gases and species with paired electrons are diamagnetic
|
All paired electrons
|
TiO2, V2O5, NaCl, C6H6 (benzene)
|
Insulator
|
|
Paramagnetic
|
Attracted by the magnetic field due to the presence of permanent magnetic dipoles (unpaired electrons). In magnetic field, these tend to orient themselves parallel to the direction of the field and thus, produce magnetism in the substances.
|
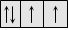
At least one unpaired electron
|
O2, Cu2+, Fe3+, TiO, Ti2O3, VO, VO2, CuO
|
Electronic appliances
|
|
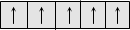
Ferromagnetic
|
Permanent magnetism even in the absence of magnetic field, Above a temperature called Curie temperature, there is no ferromagnetism.
|
Dipoles are aligned in the same direction
|
Fe, Ni, Co, CrO2
|
CrO2 is used in audio and video tapes
|
|
Antiferromagnetic
|
This arises when the dipole alignment is zero due to equal and opposite alignment.
|
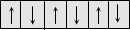
|
MnO, MnO2, Mn2O, FeO, Fe2O3; NiO, Cr2O3, CoO, Co3O4,
|
-
|
|
Ferrimagnetic
|
This arises when there is net dipole moment
|
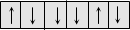
|
Fe3O4, ferrites
|
-
|
(3) Dielectric properties : The dielectric substance is that which does not permit the electricity to pass through them but on applying electric field, induced the charges are produced on its faces. In the insulator, the electrons are quite strongly held by the individual atoms. When the electric field is applied polarization takes place as the nuclei are attracted to one side and the electron cloud to other side. As a result of which, the dipoles are created. This type of crystals shows the below written properties,
(i) Piezoelectricity : Some of the crystals, the dipoles might align themselves is the ordered way so as to provide some net dipole moment. When the mechanical stress is applied in such crystals to deform them, electricity is produced because of the displacement of ions. The electricity hence produced is termed as piezoelectricity and the crystals are called as piezoelectric crystals. Its examples are, Quartz, Rochelle's salt ( sodium potassium tartarate). The Piezoelectric crystals act as the mechanical-electric transducer. These crystals are used as the pick-ups in record players where they produce the electric signals by application of the pressure.
(ii) Pyroelectricity : On heating, some of the polar crystals generate a small electric current. The electricity hence produced is called pyroelectircity.
(iii) Ferroelectricity : In some of the piezoelectric crystals, the permanent alignment of dipoles is always there even in the absence of electric field, though, on applying field the direction of the polarization changes. This phenomenon is termed as ferroelectricity and the crystals are termed as ferroelectric crystal. Example of which are, Barium titanate Potassium hydrogen phosphate (KH2PO4), (BaTiO3).
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Properties of solids questions? Properties of solids topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Properties of solids related problems. We provide step by step Properties of solids question's answers with 100% plagiarism free content. We prepare quality content and notes for Properties of solids topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours