The process of Balancing of the oxidation-reduction reactions
Though there are a number of methods for balancing oxidation - reduction reactions, two methods are very significant. These are stated below,
(1) Oxidation number method
(2) Ion - electron method
(1) Oxidation number method : The method for balancing redox reactions by oxidation number change method was developed by the scientist named Johnson. In the balanced redox reaction, over all increase in the oxidation number must be equal to the total decrease in oxidation number. This equivalence gives the basis for balancing redox reactions. This procedure is applicable to both molecular and ionic equations. The general the procedure includes the following steps,
(i) Write the skeleton equation (if not given, frame it) representing the chemical change.
(ii) Assign oxidation numbers to the atoms in the equation and find out which atoms are undergoing oxidation and reduction. Write down the separate equations for the atoms undergoing oxidation and reduction.
(iii) Find the change in oxidation number in each equation. Create the change equal in both the equations by multiplying with suitable integers. Now add both the equations.
(iv) Complete the balancing by inspection. First balance the substances which have undergone change in oxidation number and then other atoms except hydrogen and oxygen. Finally balance the hydrogen and oxygen by putting H2O molecules wherever required.
The final balanced equation should be verified to ensure that there are as many atoms of each element on the right as there are on the left.
(v) In ionic equations the net charges on both sides of the equation must be exactly the same. Use H+ ion/ions in acidic reactions and OH- ion/ions in basic reactions to balance the charge and number of hydrogen and oxygen atoms.
The below stated example illustrate the above rules,
Step : I
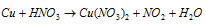 (Skeleton equation)
(Skeleton equation)
Step: II Writing down the oxidation number of all the atoms.
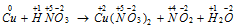
Step: III Change in the oxidation number has occurred in copper and nitrogen.

The increase in oxidation number of copper = 2 units per molecule Cu
Decrease in oxidation number of nitrogen = 1 unit per molecule HNO3
Step: IV To make the increase and decrease equal, equation (ii) is multiplied by 2.
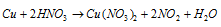
Step: V Balancing the nitrate ions, hydrogen and oxygen, following equation is obtained.
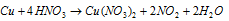
This is the balanced equation.
(2) Ion-electron method (half reaction method)
Jette and LaMev developed the method for balancing redox-reactions by ion electron method in 1927. It includes the following steps
(i) Write the redox reaction in the ionic form.
(ii) Split redox reaction into two half reactions, one for the oxidation and other for reduction.
(iii) Balance each half reaction for number of atoms of each element. For this purpose,
(a) Balance atoms other than H and O for each half reaction by making use of simple multiples.
(b) Add water molecules to side deficient in oxygen and H+ to the side deficient in hydrogen. This is performed in acidic or neutral solutions.
(c) In alkaline solution, for each excess of oxygen, add one water molecule to same side and 2OH- ions to the other side. If the hydrogen is unbalanced still, add one OH- ion for the each excess hydrogen on the same side and one water molecule to the other side.
(iv) Add the electrons to side deficient in electrons as to equalise the charge on both sides.
(v) Perform the multiplication one or both the half reactions by a suitable number so that number of electrons become equal in both the equations.
(vi) Do the addition of the two balanced half reactions and cancel any term common to both sides.
The below stated example illustrate the above rules
Step: I 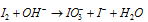 (Ionic equation)
(Ionic equation)
Step: II Splitting into two half reactions,
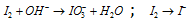 (Oxidation half reaction) (Reduction half reaction)
(Oxidation half reaction) (Reduction half reaction)
Step: III Adding OH- ions, 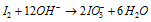
Step: IV By adding electrons to the sides deficient in electrons, (Si)
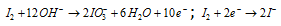
Step: V Balancing the electrons in both the half reactions.
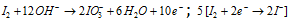
Step: VI By adding both the half reactions.
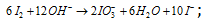
Dividing by 2, 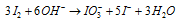
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Process of Balancing of the oxidation-reduction reaction questions? Process of Balancing of the oxidation-reduction reaction topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Process of Balancing of the oxidation-reduction reaction related problems. We provide step by step Process of Balancing of the oxidation-reduction reaction question's answers with 100% plagiarism free content. We prepare quality content and notes for Process of Balancing of the oxidation-reduction reaction topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours