Preparation of Dihydrogen : Dihydrogen can be prepared by the following methods,
(i) By action of water with metals
(a) Active metals like Na, K react at room temperature
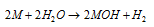 [M = Na, K etc.]
[M = Na, K etc.]
(b) Less active metals such as Zn, Ca, Mg, Al liberate hydrogen only on heating.
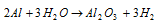
(c) Metals such as Co, Fe, Sn Ni, can react only when steam is passed over red hot metals.
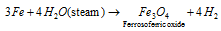
(ii) By action of water on the alkali and alkaline earth metals hydrides
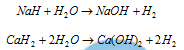
(iii) By reaction of metals like Zn, Sn, Al with alkalies (NaOH or KOH)
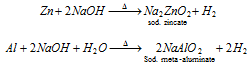 Hydrogen
Hydrogen
(iv) By action of metal with acids : All active metals which lie above hydrogen in electrochemical series, can displace hydrogen gas from dilute mineral acids like HCl, H2SO4.
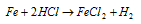
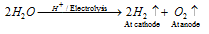
(v) By the electrolysis of acidified water
(vi) Laboratory method : In laboratory, it is obtained by action of granulated zinc with dilute H2SO4.
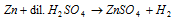
It must be noted that
(a) Pure zinc is not used for the preparation of H2 as rate of reaction of pure Zn with dil.H2SO4 is quite slow.
(b) Conc.H2SO4 is not used because then SO2 gas is evolved instead of H2.
(vii) The preparation of pure hydrogen: It may be obtained by the following steps
(a) The action of the pure diluted H2SO4 on pure magnesium ribbon.
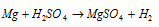
(b) Hydrogen of the high purity (> 99.95%) is obtained by the electrolysing warm aqueous barium hydroxide between nickel electrodes.
(c) By action of the water on sodium hydride.
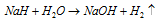
(d) By action of the KOH (aqueous) on aluminium.
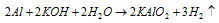
(viii) The commercial production of dihydrogen
(a) Bosch process : In this process, water gas is mixed with twice its volume of steam and passed over heated catalyst Fe2O3 in the presence of a promoter Cr2O3 or ThO2 at 773 K when CO2 and H2 are obtained. CO2 is removed by dissolving it in water under pressure (20-25 atm) and H2 left undissolved is collected.
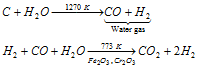
About 18% of the world's production of H2 is obtained from coal.
(b) Lane's process : By passing the steam over the spongy iron at 773-1050 K.
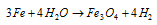
The ferrosoferric oxide (Fe3O4) so produced is reduced back to iron with water. this reaction is called as the vivification reactions
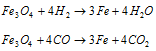
(c) By the electrolysis of water : The Electrolysis of acidified water using platinum electrodes is used for the bulk preparation of hydrogen.
(d) From hydrocarbons : Hydrocarbons (alkanes) react with steam at high temperature to produce carbon monoxide and hydrogen, for instance
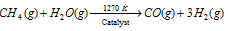
The mixture of CO and H2 so obtained can be converted into hydrogen as in Bosch process. About 77% of the world's production of H2 is obtained from hydrocarbons.
(e) It can also be produced as the by-product of the brine electrolysis process for the manufacture of Cl2 and NaOH.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Preparation of Dihydrogen questions? Preparation of Dihydrogen topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Preparation of Dihydrogen related problems. We provide step by step Preparation of Dihydrogen question's answers with 100% plagiarism free content. We prepare quality content and notes for Preparation of Dihydrogen topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours