Preparation of carbonyl compounds
(1) From alcohols (i) By oxidation.
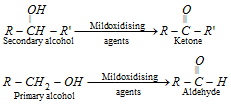
- When the secondary alcohols can be oxidised to ketones by aluminium tert-butoxide, [(CH3)3Co]3Al the reaction is known as oppenauer oxidation. Unsaturated secondary alcohols may also be oxidised to unsaturated ketones (without affecting double bond) by this reagent.
- The yield of aldehydes is usually low by these procedures. The allylic alcohols may be converted to aldehydes by treating with oxidising agent pyridinium chloro-chromate (C5H5NH+CrO3Cl-). It is abbreviated as PCC and is called Collin's reagent. This reagent is needed in non-aqueous solvents like CH2Cl2 (dichloro methane). It is prepared by mixing pyridine, CrO3 and HCl in dichloromethane. This is a very good reagent because it checks the further oxidation of aldehydes to carboxylic acids and is suitable method for preparing a,b-unsaturated aldehydes.
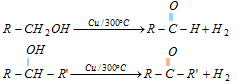
(ii) Dehydrogenation of 1° and 2° alcohols by Cu/300° or Ag/300°C.
(2) From carboxylic acids
(i) Distillation of Ca, Ba, Sr or Th salts of monobasic acids
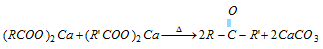
Thus in the product, one alkyl set comes from one carboxylic acid and other alkyl group from other carboxylic acid.
Calcium salts derived by dibasic acid (1, 4 and higher) on distillation give cyclic ketones.
(ii) Dehydration or Decarboxylation of acids by MnO/300°C.
(a) This reaction takes place between two molecules of carboxylic acids. Both can be the similar or different.
(b) If one of the carboxylic acids is HCOOH then this acid undergoes decarboxylation because this acid is the only monobasic acid which undergoes decarboxylation even in the absence of catalyst.
Case I: When both molecules are HCOOH
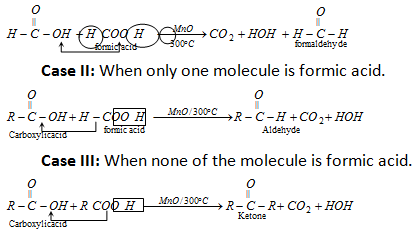
(3) From alkenes
(i) Ozonolysis : Alkenes on reductive ozonolysis give carbonyl compounds
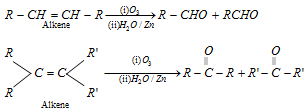
q This method is used only for aliphatic carbonyl compounds.
(ii) Oxo process
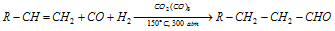
- Oxo process is used only for the preparation of aldehydes.
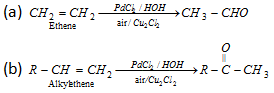
(iii) Wacker process
(4) From Grignard reagents
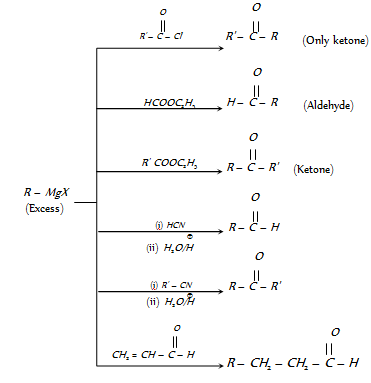
(5) From cyanides
(i) Stephen aldehyde synthesis : Conversion of cyanides into aldehydes by partial reduction with SnCl2/HCl, followed by hydrolysis, is known as Stephens aldehyde synthesis.
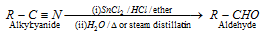
(Only used for aldehydes)
(6) From Alkyl halides and benzyl halides
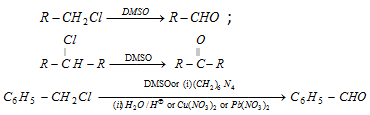
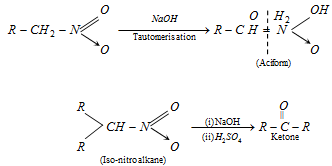
(7) From nitro alkanes : Nitro alkanes having at least one a -hydrogen atom give carbonyl compounds on treatment with conc NaOH followed by 70% H2SO4. The reaction is known as Nef carbonyl synthesis.
(8) Reaction with excess of alkyl lithium : Carboxylic acids react with excess of organo lithium compound to give lithium salt of gem diols which on hydrolysis give ketones.
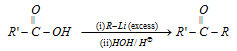
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Preparation of Carbonyl Compounds questions? Preparation of Carbonyl Compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Preparation of Carbonyl Compounds related problems. We provide step by step Preparation of Carbonyl Compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for Preparation of Carbonyl Compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours