General processes of preparation of Alkyl Halides
(1) From alkanes
(i) By halogenation :
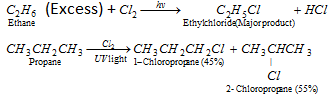
This reaction tends to free radical mechanism.
- Order of reactivity of X2 for a provided alkane is, F2 > Cl2 > Br2 > I2.
- The reactivity of the alkanes follows the sequence :
3°alkane > 2°alkane > 1°alkane.
(ii) With sulphuryl chloride :
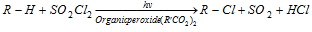
- This reaction is a fast due to in presence of light and trace of an organic peroxide.
(2) From alkenes (Hydrohalogenation by Electrophillic addition)
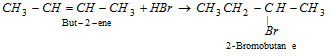
- Addition of HBr to alkene in the existence of organic peroxide take place due to peroxide effect or Kharasch's effect.
- This addition take place by two methods, Peroxide initiates free radical mechanism.
- Markownikoff's addition by electrophillic mechanism.
- The sequence of reactivity of halogen acids is, HI > HBr > HCl.
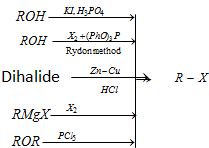
(3) From alcohols
(i) By the action of halogen acids
Groove's process
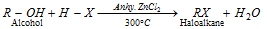
- The reactivity sequence of HX in the above reaction is : HI > HBr > HCl > HF.
- Reactivity order of alcohols 30 > 20 > 10 > MeOH.
- 2° and 3° alcohols undergo SN1; whereas 1° and MeOH undergo SN2 mechanism.
- Concentrated HCl + anhy. ZnCl2 is known as lucas reagent.
(ii) Using PCl5 and PCl3 :
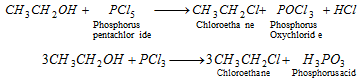
- Bromine and iodine derivatives cannot be obtain from the above reaction, because PBr5 or PI5 are unstable.
- This method gives good yield of primary alkyl halides but poor yields of secondary and tertiary alkyl halides.
(iii) By the action of thionyl chloride (Darzan's process) : Reaction takes place through SN2 mechanism.
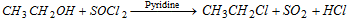
(4) From silver salt of carboxylic acids (Hunsdiecker reaction, Decarboxylation by Free radical mechanism)
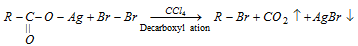
- The reactivity of alkyl group is 10 > 20 > 30.
- Only bromide are found in good yield.
- Not suitable for chlorination due to yield is poor.
- In this reaction iodine forms ester instead of alkyl halide and the reaction is called Birnbourn-Simonini reaction,
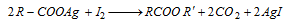 .
.
(5) By Finkelstein reaction (Halide exchange method) : 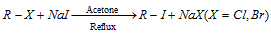
r Alkyl fluorides cannot be prepared by that process. They may be found from corresponding chlorides by the action of Hg2F2 or antimony trifluoride.
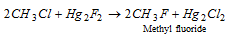
(6) Other method
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Preparation of Alkyl Halides questions? Preparation of Alkyl Halides topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their XPreparation of Alkyl Halides related problems. We provide step by step Preparation of Alkyl Halides question's answers with 100% plagiarism free content. We prepare quality content and notes for Preparation of Alkyl Halides topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours