Manganese containing compound
Potassium Permanganate, (KMnO4)
Potassium permanganate is a salt of an unstable acid HMnO4 (permanganic acid). The Mn is an +7 state in this compound.
Preparation : Potassium permanganate is obtained from pyrolusite as follows.
The conversion of pyrolusite to potassium manganate : When the manganese dioxide is fused with potassium hydroxide in presence of air or an oxidising agent like potassium nitrate or chlorate, potassium manganate is formed, possibly by the help of potassium manganite.
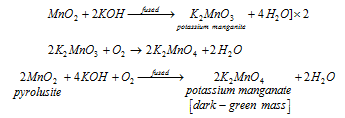
The oxidation of potassium manganate to potassium permanganate : The potassium manganate so obtained is oxidised to potassium permanganate by either of the following methods.
By chemical method : The fused dark-green mass is extracted with a small quantity of water. Filtrate is warmed and treated with current of the ozone, chlorine or carbon dioxide. Potassium manganate gets oxidised to potassium permanganate and the hydrated manganese dioxide precipitates out. The reactions taking place are shown as follows,
When CO2 is passed
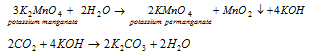
When chlorine or ozone is passed
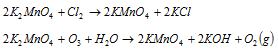
Purple solution obtained by this is concentrated and dark purple, needle-like crystals having metallic lustre are obtained.
Electrolytic method : Presently, potassium manganate (K2MnO4) is oxidised electrolytically. The reactions at electrode are,
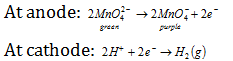
Purple solution containing the KMnO4 is evaporated under the controlled condition to obtain crystalline sample of potassium permanganate.
Physical properties
KMnO4 crystallizes as the dark purple crystals with greenish luster (melting point 523 K).
It is soluble in water to an extent of 6.5grams per 100grams at room temperature. The aqueous solution of the KMnO4 has purple colour.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Potassium Permanganate questions? Potassium Permanganate topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Potassium Permanganate related problems. We provide step by step Potassium Permanganate question's answers with 100% plagiarism free content. We prepare quality content and notes for Potassium Permanganate topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours