The Construction of structures for molecules and poly atomic ions : The below written method is applicable to the species in which the octet rule is not violated.
(i) To determine the total number of valence electrons in all atoms present, including the net charge on the species (n1).
(ii) To determine n2 = [2 × (number of H atoms) + 8 × (number of other atoms)].
(iii) Determine the number of bonding electrons, n3, which equals n2 - n1. No. of bonds equals n3/2.
(iv) Determine the number of non-bonding electrons, n4, which equals n1 - n3. No. of lone pairs equals n4/2.
(v) Knowing the central atom (you'll need to know some chemistry at this point, as math will not help hear!), arrange and distribute other atoms and n3/2 bonds. Then the complete octets making use of the n4/2 lone pairs.
(vi) Establish the 'formal charge' on each atom.
(vii) The Formal Charge = [valence electrons present in the atom) - (number of bonds) - (number of unshared electrons)]
(viii) Other aspects such as resonance and many athers can now be incorporated.
Illustrative examples
(i) 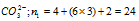 [2 added for net charge]
[2 added for net charge]
n2 = (2 × 0) + (8 × 4) = 32 (no. H atom, 4 other atoms (1'C' and 3 'O')
n3 = 32 - 24 = 8, hence 8/2 = 4 bonds
n4 = 24 - 8 = 16, hence 8 lone pairs.
Since carbon is the central atom, 3 oxygen atoms are to be arranged around it, thus, 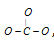 , but total bonds are equal to 4.
, but total bonds are equal to 4.
Hence, we get 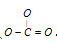 . Now, arrange lone pairs to complete octet
. Now, arrange lone pairs to complete octet 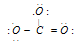
(ii) n1 = 4 + (6 × 2 ) = 16
n2 = (2 × 0) + (8 × 3) = 24
n3 = 24 - 16 = 8, hence 4 bonds
n4 = 16 - 8 = 8, hence 4 lone-pairs
Since C is the central atom hear, the two oxygen atoms are around to be arranged it hence the structure would be; O - C - O, but total no. of bonds = 4
Thus, O = C = O. After arrangement of lone pairs to complete octets, we get,  and hence final structure is
and hence final structure is 
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Polyatomic ions questions? Polyatomic ions topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Polyatomic ions related problems. We provide step by step Polyatomic ions question's answers with 100% plagiarism free content. We prepare quality content and notes for Polyatomic ions topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours