Physical properties of Ether
(1) Physical state : Methoxy methane and methoxy ethane are gases while other members are volatile liquid with pleasant smell.
(2) Dipole moment (D.M.) : Bond angle of ether is due to sp3 hybridisation of oxygen molecules. Since C - O bond is a polar covalent bond, hence ether possess a total dipole moment, even if they are regular. Dipole moment of dipole moment of di ethyl ether is 1.18 Dand dimethyl ether is 1.3 D.
- The bigger bond angle may be because of greater repulsive interaction between bulkier alkyl groups as compared to smaller H-atoms in water.
(3) Boiling points : Boiling points of ethers are much lower than those of isomeric alcohols, but nearer to alkanes having comparable volume. This is because of to the absence of hydrogen bonding in ethers.
(4) Solubility : Solubilities of ethers in water are comparable with those of alcohols.
Example : Di ethyl ether and n-butyl alcohol have approximately the same solubility in water. This is cause, ether form hydrogen covalent bond with water much in the same way as alcohol do with water.
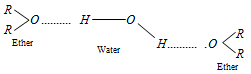
q Solubility of ether in water reduces with the size of alkyl groups.
(5) Hydrogen bonding : There is no hydrogen directly attach (bonded) to oxygen in ethers, so ethers do not show any intermolecular hydrogen bonding.

(6) Density : Ethers are lighter than water.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical Properties of Ether questions? Physical Properties of Ether topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical Properties of Ether related problems. We provide step by step Physical Properties of Ether question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical Properties of Ether topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours