Physical characteristics of carbonyl compounds
(1) Physical state : Methanal is a pungent smell gas. Ethanal is a volatile liquid, boiling points 294 K. Other aldehydes and ketones containing up to eleven carbon atoms are colourless liquids while higher members are solids.
(2) Smell : With the exception of lower aldehydes which have unpleasant odours, aldehydes and ketones have generally pleasant smell. As the size of the atom increases, the odour goes less pungent and more fragrant. In fact, several naturally happening aldehydes and ketones have been used in blending of perfumes and flavouring agents.
(3) Solubility : Aldehydes and ketones upto four carbon atoms are miscible with water. This is due to the presence of hydrogen bonding between the polar carbonyl group and water molecules as shown below :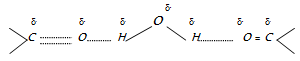
With the increase in the size of alkyl group, the solubility decreases and the compounds with more than four carbon atom are practically insoluble in water. All aldehydes and ketones are, however, soluble in organic solvents such as ether, alcohol, etc. The ketones are good solvents themselves.
(4) Boiling points : The boiling points of aldehydes and ketones are higher than those of non polar compounds (hydrocarbons) or weakly polar compounds (such as ethers) of comparable molecular masses. However, their boiling points are lower than those of corresponding alcohols or carboxylic acids. This is because aldehydes and ketones are polar compounds having sufficient intermolecular dipole-dipole interactions between the opposite ends of C = O dipoles.
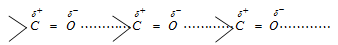
However, these dipole-dipole interactions are weaker than the intermolecular hydrogen bonding in alcohols and carboxylic acids. Therefore, boiling points of aldehydes and ketones are relatively lower than the alcohols and carboxylic acids of comparable molecular masses.
Among the carbonyl compounds, ketones have slightly higher boiling points than the isomeric aldehydes. This is due to the presence of two electrons releasing groups around the carbonyl carbon, which makes them more polar.

(5) Density : Density of aldehydes and ketones is less than that of water.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical Properties of Carbonyl Compounds questions? Physical Properties of Carbonyl Compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical Properties of Carbonyl Compounds related problems. We provide step by step Physical Properties of Carbonyl Compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical Properties of Carbonyl Compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours