Physical properties
(i) CH3F, CH3Cl, CH3Br and C2H5Cl are gases at room temperature. The alkyl halides upto C18 are colourless liquids while higher members are colourless solids.
(ii) Alkyl halides are not soluble in water but soluble in organic solvents.
(iii) They burn on copper wire with green outer flame (Beilstein test for halogens).
(iv) Alkyl bromides and iodides are heavier than water. Alkyl fluorides and chlorides are lighter than water.
(v) Alkyl iodides become violet or brown in colour on exposure as they decompose in light.
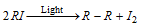
(vi) For a provided alkyl set, the boiling points of alkyl halides are in the order RI > RBr > RCl > RF and for a given halogen the boiling points of alkyl halides increase with the increase of the size of the alkyl group.
(vii) Alkyl halides are in usual toxic elements and bring unconsciousness when inhaled in large amounts.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical Properties of Alkyl Halides questions? Physical Properties of Alkyl Halides topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical Properties of Alkyl Halides related problems. We provide step by step Physical Properties of Alkyl Halides question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical Properties of Alkyl Halides topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours