Physical properties
(1) Physical state : All are greyish- light, white, malleable and ductile metals with metallic luster. Their hardness decrease progressively with increase in the atomic number. Though these are fairly soft but relatively harder than the alkali metals.
(2) Atomic and ionic radii
(i) The atomic and ionic radii of alkaline earth metals also increase down the group due to progressive addition of new energy shells like alkali metals.
Be Mg Ca Sr Ba Ra
Atomic radius (pm) 112 160 197 215 222 -
Ionic radius of M2+ 31 65 99 113 135 140
ion (pm)
(ii) The atomic radii of alkaline earth metals are however smaller than their corresponding alkali metal of the same period. This is because of the fact that alkaline earth metals possess a higher nuclear charge than alkali metals which more effectively pulls the orbit electrons towards the nucleus causing a decrease in size.
(3) Density
(i) Density decreases slightly upto Ca after which it increases. Decrease in the density from Be to Ca may be due to less packing of atoms in solid lattice of Mg and Ca.
Be Mg Ca Sr Ba Ra
1.84 1.74 1.55 2.54 3.75 6.00
(ii) The alkaline earth metals are more heavier, denser, and harder than the alkali metal. The higher density of the alkaline earth metals is because of their smaller atomic size and strong intermetallic bonds which provide a more close packing in crystal lattice as compared to alkali metals.
(4) Melting point and Boiling point
(i) The melting points and boiling points of the alkaline earth metals do not show any regular trend.
Be Mg Ca Sr Ba Ra
melting points (K) 1560 920 1112 1041 1000 973
boiling point (K) 2770 1378 1767 1654 1413 -
(ii) The values are, though, more than alkali metals. This can be due to close packing of atoms in crystal lattice in alkaline earth metals.
(5) Ionisation energy and electropositive or metallic character
(i) Since the atomic size decreases along the period and the nuclear charge increases and thus the electrons are more tightly held towards nucleus. It is hence alkaline earth metals have higher ionisation energy in comparison to the alkali metals but lower ionisation energies in comparison to the p-block elements.
(ii) The ionisation energy of the alkaline earth metals decreases from Be to Ba.
Be Mg Ca Sr Ba Ra
First ionisation energy (k J mol-1) 899 737 590 549 503 509
Second ionisation energy (kJ mol-1) 1757 1450 1146 1064 965 979
(iii) The higher values of the second ionisation energy is because of the fact that removal of one electron from valence shell, the remaining electrons are more tightly held in which the nucleus of cation and hence more energy is needed to pull one more electron from the monovalent cation.
(iv) No doubt first ionisation energy of alkaline earth metals are higher than alkali metals but a closer look on 2nd ionisation energy of alkali metals and alkaline earth metals reveals that 2nd ionisation energy of alkali metals are more
Li Be
1st ionisation energy (kJ mol-1) 520 899
2nd ionisation energy (kJ mol-1) 7296 1757
This may be explained as,
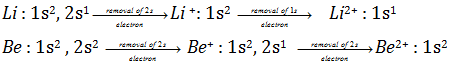
The removal of 2nd electron from alkali metals takes place from 1s sub shell which are more closer to nucleus and exert more nuclear charge to hold up 1s electron core, while removal of 2nd electron from alkaline earth metals takes from 2s sub shell. Closer are shells to the nucleus, more tightly will be held electrons with the nucleus and thus more energy is needed to remove the electron.
(v) All these have strong electropositive character which increases from Be to Ba.
(vi) These posses less electropositive character than alkali metals as the later have low values of the ionisation energy.
(6) Oxidation number and valency
(i) The IE1 of these metals are much lower than IE1 and therefore it appears that these metals should form univalent ion rather than divalent ions but in actual practice, all these provide bivalent ions. This is because of the fact that M2+ ion possesses a higher degree of hydration or M2+ ions are extensively hydrated to form the [M(H2O)x]2+, a hydrated ion. This includes a large amount of energy evolution which counter balances the higher value of second ionisation energy.
M → M2+ , DH = IE1 + E2
M2+ + xH2O → [M(H2O)x]2+; DH = - hydration energy.
(ii) The tendency of these metals to exist as divalent cation can hence be accounted as,
(a) Divalent cation of these metals have noble gas or stable configuration.
(b) Formation of the divalent cation lattice leads to the evolution of energy because of strong lattice structure of divalent cation which easily compensates for higher values of second ionisation energy of these metals.
(c) Higher heats of the hydration of divalent cation which accounts for the existence of the divalent ions of these metals in solution state.
(7) Hydration of ions
(i) The hydration energies of alkaline earth metals divalent cation are much more than the hydration energy of monovalent cation.
Mg+ Mg2+
Hydration energy or Heat of hydration (kJ mol-1) 353 1906
The abnormally higher values of heat of hydration for divalent cations of alkaline earth metals are responsible for their divalent nature. MgCl2 formation occurs with more amount of heat evolution and thus MgCl2 is more stable.
(ii) The hydration energies of M2+ ion decreases with increase in ionic radii.
Be2+ Mg2+ Ca2+ Sr2+ Ba2+
Heat of hydration kJ mol-1 2382 1906 1651 1484 1275
(iii) Heat of the hydration are larger than the alkali metals ions and thus alkaline earth metals compounds are more extensively hydrated than those of alkali metals e.g MgCl2 and CaCl2 exists as Mg Cl2 .6H2O and CaCl2. 6H2O which NaCl and KCl do not form such hydrates.
(iv) The ionic mobility, thus, increases from Be2+ to Ba2+, as the size of hydrated ion decreases.
(8) Electronegativities
(i) The electronegativities of alkaline earth metals are also small but are higher than alkali metals.
(ii) Electronegativity decreases from Be to Ba as shown below,
Be Mg Ca Sr Ba
Electronegativity 1.57 1.31 1.00 0.95 0.89
(9) Conduction power : Good conductor of heat and electricity.
(10) Standard oxidation potential and reducing properties
(i) The standard oxidation potential (in volts) are,
Be Mg Ca Sr Ba
1.69 2.35 2.87 2.89 2.90
(ii) All these metals possess tendency to lose two electrons to give M2+ ion and are used as reducing agent.
(iii) The reducing character increases from Be to Ba, though, these are less powerful reducing agent than alkali metals.
(iv) Beryllium possesing relatively lower oxidation potential and thus does not liberate H2 from acids.
(11) Characteristic flame colours
The characteristic flame colour shown are : Ca - brick red; Sr -crimson ; Ba-apple green and Ra- crimson.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical Properties of Alkaline Earth Metals questions? Physical Properties of Alkaline Earth Metals topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical Properties of Alkaline Earth Metals related problems. We provide step by step Physical Properties of Alkaline Earth Metals question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical Properties of Alkaline Earth Metals topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours