Physical properties
(1) Physical state
(i) All these are silvery white, soft and light solids. All these can be cut with the help of knife. When freshly cut, they posses bright lustre which quickly tarnishes because of surface oxidation.
(ii) These form diamagnetic colourless ions as these ions do not have unpaired electrons, (that is M+ has ns0configuration). This is why alkali metal salts are colourless and diamagnetic in nature.
(2) Atomic and ionic radii
(i) The alkali metals have largest atomic and ionic radii than their successive elements of other groups belonging to same period.
(ii) The atomic and ionic radii of alkali metals, though, increases down the group because of progressive addition of new energy shells.
No doubt that the nuclear charge also increases on moving down group but the influence of the addition of energy shell predominates
Li Na K Rb Cs Fr
Atomic radius (pm) 152 186 227 248 265 375
Ionic radius of M+ 60 95 133 148 169 -
ions (pm)
(3) Density
(i) All these are light metals, Li, Na and K have density less than that of water. Low values of the density are because these metals have high atomic volume due to larger atomic size. When we move down the group the atomic size as well as atomic mass both increase but increase in atomic mass predominates over increase in atomic size or atomic volume and therefore the ratio mass/volume that is density gradually increases down the groups
(ii) The density increases at slowly as we go Li to Cs, Li is lightest known metal among all of them.
Li = 0.534, Na = 0.972, K = 0.86, Rb = 1.53 and Cs = 1.87 g/ml at 200C.
(iii) K is lighter than Na due to its unusually large atomic size.
(iv) In solid state, they posses body centred cubic lattice.
(4) Melting point and Boiling point
(i) All these elements have low melting point and boiling point in comparison to the other group members.
Li Na K Rb Cs Fr
melting point (K) 453.5 370.8 336.2 312.0 301.5 -
boiling point (K) 1620 1154.4 1038.5 961.0 978.0 -
(ii) Lattice energy of these atoms in the metallic crystal lattice relatively low because of larger atomic size and hence possess low melting point and boiling point on moving down the group, atomic size increases and binding energy of their atoms in the crystal lattice decreases which results lowering of the melting point.
(iii) The lattice energy decreases from Li to Cs and hence melting point and boiling point also decreases from Li to Cs.
(5) Ionisation energy and electropositive or metallic character
(i) Due to unpaired lone electron in ns sub-shell as well as due to the larger size of them, the outermost electron is far from the nucleus, the removal of the electron is much easier and these low values of ionisation energy. (I.E.)
(ii) Ionisation energy of these metal decreases from Li to Cs.
Ionisation energy Li Na K Rb Cs Fr
IE1 520 495 418 403 376 -
IE2 7296 4563 3069 2650 2420 -
A jump in 2nd ionisation energy (huge difference) can be explained as,
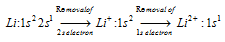
Removal of 1s electrons from Li+ and that too from completely filled configuration requires much more energy and a jump in 2nd ionisation is noticed.
(iii) Lower are ionisation energy values, greater is tendency to lose the ns1 electron to change in M+ ion (i.e. M ® M++e-) and therefore stronger is electropositive character.
(iv) Electropositive character increases from Li to Cs.
Due to their strong electropositive nature, they release electrons even when exposed to light showing photoelectric effect. The property of it is responsible for use of the Cs and K in photoelectric cell.
(6) Oxidation number and valency
(i) Alkali metals are univalent in nature due to low ionisation energy values and form ionic compounds. Lithium salts are, although, covalent.
(ii) Further, the M+ ion acquires the stable noble gas configuration. It needs high values of energy to pull out another electron from next to outer shell of M+ ion and that is why their second ionisation energy is very high. As a result, under ordinary conditions, it is impossible for these metals to form the M2+ ion and thus they show +1 oxidation state.
(iii) As the electronic configuration of M+ ions do not have unpaired electron and hence alkali metal salts are diamagnetic and colourless. Those alkali metal salts are coloured which posses coloured anions such as K2Cr2O7 is orange because of orange coloured Cr2O72- ion, KMnO4 is violet due to violet coloured MnO41- ion.
(7) Hydration of Ions
(i) Hydration represents for dissolution of the substance in water to get adsorb water molecule by the weak valency force. Hydration of the ions is the exothermic process (which means energy is released during the hydration) when ions on dissolution water get hydration.
(ii) The energy released when 1 mole of the ion in gaseous state is dissolved in water to get it hydrated is termed as hydration energy
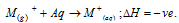
(iii) The Smaller the cation, the greater is the degree of hydration. Hydration energy is in order of, Li+ > Na+ > K+ > Rb+ > Cs+
(iv) Li+ being smallest in size has maximum degree of hydration and that is why lithium salts are mostly hydrated, LiCl. 2H2O also lithium ion being heavily hydrated, moves quite slowly under influence of the electric field and, thus, is the poorest conductor current among alkali metals ions It can, hence, be concluded that it is the degree of hydration as well as the size of the ion is responsible for current carried by the ion.
Relative ionic radii Cs+ > Rb+ > K+ > Na+ > Li+
Relative hydrated ionic radii Li+ > Na+ > K+ > Rb+ > Cs+
Relative conducting power Cs+ > Rb+ > K+ > Na + > Li+
(8) Electronegativity, Electro positivity and metallic character.
(i) These metals are highly electropositive and thereby possess low values of electronegativities. The metallic nature and electro positivity increase from the Li to Cs (Li < Na < K < Rb < Cs)
(ii) Electronegativity of alkali metals decreases down the group as the trend of numerical values of electronegativity given below on Pauling scale suggests.
Li Na K Rb Cs Fr
Electron-ve 0.98 0.93 0.82 0.82 0.79 -
Fr being radioactive elements and thus studies on physical properties of this element are limited.
(9) Specific heat : It decreases from Li to Cs.
Li Na K Rb Cs Fr
Specific heat (Cal/g) 0.94 0.293 0.17 0.08 0.049 -
(10) Conduction power : All are good conductors of heat and electricity, because of loosely held valence electrons.
(11) Standard oxidation potential and reduction properties
(i) As alkali metals simply lose ns1 electron and hence they have high values of oxidation potential that is
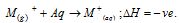
(ii) The standard oxidation potentials of the alkali metals (which is in volts) are listed below,
Li Na K Rb Cs
+3.05 +2.71 +2.93 +2.99 +2.99
(iii) The more is oxidation potential, more will be the tendency to get oxidized and hence more powerful is reducing nature in the aqueous medium that is why alkali metals liberate H2 from H2O and HCl.
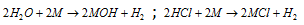
(iv) Although, an examination of the ionisation energy for alkali metals reveals that Li must possess the minimum tendency to lose the electron and thus its reducing nature must be minimum. Greatest reducing nature of the Li in aqueous medium is accounted because of the maximum hydration energy of Li+ ion. Such as for Lithium
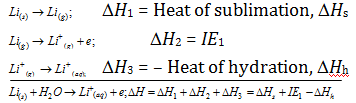
Similarly, for sodium,
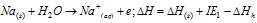
ΔHh for Li > ΔHh for Na. Therefore, large negative ΔH values are observed in case of Li and this explains for more possibility of Li to get itself oxidized or have reducing nature.
(12) Characteristic flame colours : The alkali metals and their salts give characteristic color to Bunsen flame. The flame energy creates and excitation of the outermost electron which on reverting back to its initial position gives out the absorbed energy as visible light. These color differ from each other Li -crimson, Na-Golden yellow, K - Pale violet , Rb-Red violet and Cs -Blue violet. These different colors are due to different ionisation energy of alkali metals. The energy released is least in case of the Li+ and increases in the order.
Energy released : Li+ < Na+ < K+ < Rb+ < Cs+
l released : Li+ > Na+ > K+ > Rb+ > Cs+
Frequency released : Li+ < Na+ < K+ < Rb+ < Cs+
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical Properties of Alkali Metals questions? Physical Properties of Alkali Metals topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical Properties of Alkali Metals related problems. We provide step by step Physical Properties of Alkali Metals question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical Properties of Alkali Metals topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours