Physical properties : Water is colourless, odourless and tasteless liquid at ordinary temperature.
At 273K water is in equilibrium with ice and vapour this point is known triple point.
Table: Some physical constants of H2O and D2O at 298 K
|
Constant
|
Ordinary water H2O
|
Heavy water D2O
|
|
Molecular mass
|
18.015
|
20.028
|
|
Maximum density (g cm-3)
|
1.000
|
1.106
|
|
Melting point (K)
|
273.2
|
276.8
|
|
Boiling point (K)
|
373.2
|
374.4
|
|
Heat of fusion
(KJ mol-1) at 273K
|
6.01
|
6.28
|
|
Heat of vaporisation
(KJ mol-1) at 373K
|
40.66
|
41.61
|
|
Heat of formation (KJ mol-1)
|
- 285.9
|
- 294.6
|
|
Ionisation constant
|
1.008 * 10-14
|
1.95 -* 10-15
|
(4) Chemical properties : Water shows a versatile chemical behaviour. This behaves as a base, an acid, an oxidant, a reductant and as the ligand to metals.
(i) Dissociation of water : Water is very stable and does not dissociate into its elements even at the high temperatures. The pure water has less but measurable electrical conductivity and it dissociates as shown in the reaction, 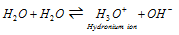
Kw = 1.0 * 10-14 mol2 L2 at 298K
(ii) Amphoteric nature : Water can act both as an acid and a base and is said to be amphoteric. Though, water is neutral towards the litmus and its pH is 7.
(iii) Oxidising and reducing nature : Water can act both as an oxidising and a reducing agent in its chemical reactions. The examples are given below
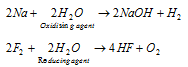
(iv) Hydrolytic reactions : Water can hydrolyse many oxides, phosphides, nitrides, carbonates halides, hydrides, carbides, etc. to give an acid or a base or both as shown below :
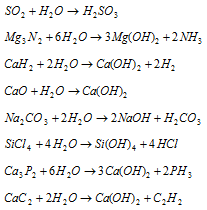
(v) Water forms the hydrates with metal salts : There are three basic types of hydrates.
(a) The compounds in which water molecule are co-ordinated to metal ion (such as complex compounds) [Ni(OH2)](NO3)2], [Fe(OH2)6]Cl3 etc.
(b) The Compound in which water molecule can be hydrogen bonded to oxygen to form the oxo-anion. For instance in CuSO4.5H2O, 4 molecules of water are co-ordinated to Cu2+ while fifth molecule is hydrogen bonded to SO42- ion.
(c) In some of the compounds, water molecule occupies, interstitial sites in the crystal lattice such as BaCl2.2H2O.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical and Chemical properties of Water questions? Physical and Chemical properties of Water topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical and Chemical properties of Water related problems. We provide step by step Physical and Chemical properties of Water question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical and Chemical properties of Water topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours