Physical properties
(i) The pure hydrogen peroxide is the pale blue syrupy liquid.
(ii) It freezes at - 0.5°C and has density of 1.4 in the pure state.
(iii) Hydrogen peroxide is diamagnetic.
(iv) It is highly associated by means of hydrogen bonding than water.
(v) Although it is a better polar solvent than H2O. However, it can't be used as such because of strong autooxidation ability.
(vi) Dipole moment of H2O2 is 2.1 D.
Chemical properties
(i) Decomposition : Pure H2O2 is an unstable liquid and decomposes into water and O2 either upon standing or upon heating,

(ii) Oxidising nature : It is a powerful oxidising agent. This acts as an oxidising agent in neutral, acidic or in alkaline medium. For example  [In the neutral medium]
[In the neutral medium]
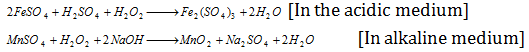
(iii) Reducing nature : H2O2 has tendency to take up oxygen from strong oxidising agents and thus, acts as a reducing agent,  It can act as a reducing agent in basic, acidic, or even neutral medium.
It can act as a reducing agent in basic, acidic, or even neutral medium.
In acidic medium,
In alkaline medium,
(iv) Bleaching action : H2O2 acts as a bleaching agent due to the release of nascent oxygen.
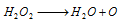
Thus, the bleaching action of H2O2 is due to oxidation. This oxidises colouring matter to the colourless product, the colouring matter +O → Colour less material.
H2O2 is used to bleach delicate materials like ivory, silk, wool, leather etc.
(v) Acidic nature : Anhydrous hydrogen peroxide is acidic in character (Ka = 1.55 * 10-12 at 298 K). Its dissociation in aqueous solution may be given as
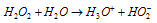
It forms two types of salts
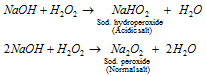
(vi) Addition reactions : Hydrogen peroxide is capable of adding itself to ethylenic linkage.
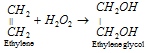
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical and Chemical properties of Hydrogen Peroxide questions? Physical and Chemical properties of Hydrogen Peroxide topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical and Chemical properties of Hydrogen Peroxide related problems. We provide step by step Physical and Chemical properties of Hydrogen Peroxide question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical and Chemical properties of Hydrogen Peroxide topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours