Physical properties of dihydrogen : It is a tasteless colourless, and odourless gas. It is lightly soluble in the water. It is extremely combustible. The Physical constants of the atomic hydrogen are as follows,
Atomic radius (pm) - 37
Ionic radius of H- ion (pm) - 210
Ionisation energy (KJ mol-1) - 1312
Electron affinity (KJ mol-1) -72.8
Electronegativity - 2.1
Chemical properties of dihydrogen : The dihydrogen is much stable and dissociates into the hydrogen atoms only when heated above the temperature of 2000 K,  . Its bond the dissociation energy is quite high,
. Its bond the dissociation energy is quite high,  . Because of its high bond dissociation energy, it is not much reactive. Although, it combines with several elements or compounds.
. Because of its high bond dissociation energy, it is not much reactive. Although, it combines with several elements or compounds.
(i) Action with metals : To forms the corresponding hydrides. 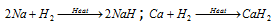 .
.
With the transition metals (elements of the d - block) such as Pd, Ni, Pt etc. dihydrogen forms the interstitial hydrides in which the small molecules of the dihydrogen takes the interstitial sites in crystal lattices of these hydrides. As a result of which the formation of interstitial hydrides, these metals adsorb very large volume of hydrogen on their surface. This property of the adsorption of the gas by a metal is known as occlusion. The occluded hydrogen may be liberated from metals by the strong heating.
(ii) Reaction with Non-metals
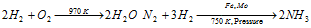
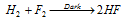
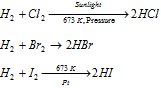
The reactivity of the halogen towards dihydrogen decreases as folows, F2 > Cl2 > Br2 > I2
As a result, F2 reacts in the dark, Cl2 in presence of the sunlight, Br2 reacts only upon heating while reaction with I2 occurs in presence of the catalyst.
(iii) Reaction with the unsaturated hydrocarbons : H2 reacts with the unsaturated hydrocarbons like ethylene and acetylene to provide saturated hydrocarbons.
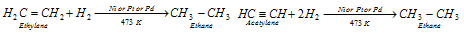
This reaction is used in hydrogenation or the hardening of oils. The vegetable oils like groundnut oil or cotton-seed oil are unsaturated in the nature because they comprise at least one double bond in their molecules. Dihydrogen is passed through oils at about temperature of 473 K in the presence of the catalyst to form solid fats. The vegetable ghee like Dalda, Rath, etc. is commonly prepared by this process.
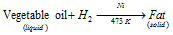
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical and Chemical properties of Dihydrogen questions? Physical and Chemical properties of Dihydrogen topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical and Chemical properties of Dihydrogen related problems. We provide step by step Physical and Chemical properties of Dihydrogen question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical and Chemical properties of Dihydrogentopic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours