Physical properties of aromatic nitro compounds
(i) Aromatic nitro compounds are insoluble in water but soluble in organic solvents.
(ii) They are either pale yellow liquids or solids having distinct smells. For example, nitro benzene (oil of Mirabane) is a pale yellow liquid having a smell of bitter almonds.
Chemical properties of aromatic nitro compounds
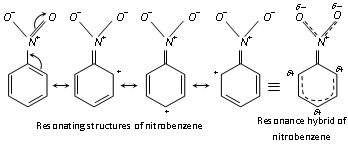
(i) Resonance in nitrobenzene imparts a partial double bond character to the bond between carbon of benzene nucleus and nitrogen of the - NO2 group with the result the - NO2 group is firmly bonded to the ring and therefore cannot be replaced other groups, i.e., it is very inert.
(ii) Displacement of the - NO2 group : Although - NO2 group of nitrobenzene cannot be replaced by other groups, but if a second - NO2 group is present on the benzene ring of nitrobenzene in the o- or p- position, it can be replaced by a nucleophile. For example,
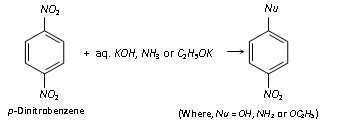
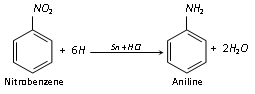
(iii) Reduction : Aromatic nitro compounds can be reduced to a variety of product as shown below in the case of nitrobenzene.
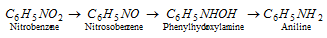
The nature of the final product depends mainly on the nature (acidic, basic or neutral) of the reduction medium and the nature of the reducing agent.
(a) Reduction in acidic medium
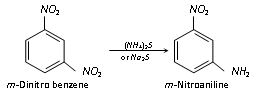
Reduction of dinitrobenzene with respect to ammonium sulphide reduces only one - NO2 group (selective reduction)
(b) Reduction in neutral medium :
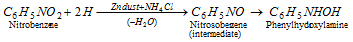
(c) Reduction in alkaline medium :
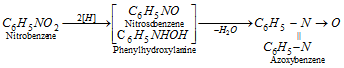
Azoxybenzene on further reduction yields azobenzene and hydrazobenzene.
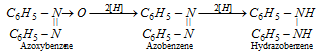
(d) Electrolytic reduction :
· Weakly acidic medium of electrolytic reduction gives aniline.
· Strongly acidic medium gives phenylhydroxylamine which rearranges to p-aminophenol.

· Alkaline medium of electrolytic reduction gives all the mono- and di-nuclear reduction products mentioned above in point (c).
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Physical and Chemical properties of aromatic nitro compounds questions? Physical and Chemical properties of aromatic nitro compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Physical and Chemical properties of aromatic nitro compounds related problems. We provide step by step Physical and Chemical properties of aromatic nitro compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for Physical and Chemical properties of aromatic nitro compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours