Photography:
The photographic plate is covered with a colloidal gelatinised solution of AgBr. When, the exposure, AgBr is decreased to the metallic silver.
This is described in the following equation:
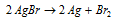
The exposed film is developed. The developer employed is an alkaline solution of hydroquinone or quinol that reduces a number of the exposed AgBr to black silver.
Equation:
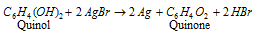
The film is finally fixed by dipping in a solution of sodium thiosulphate or hypo that eliminates not changed AgBr like complex ion.
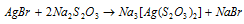
Later than taking the print of photograph at last it is toned by dipping in a dilute solution of gold chloride to impart a beautiful golden colour or it is dipped in potassium chloro platinate K2PtCl6 solution to obtain a shining grey tinge.
Equation for this is as shown below:
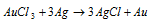
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Photography questions? Photography topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Photography related problems. We provide step by step Photography question's answers with 100% plagiarism free content. We prepare quality content and notes for Photography topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours