Phases of the colloids and their classification
(1) Phases of colloids: We know that the colloidal solution is of the heterogeneous nature. It comprises of two phases which are as follows
(i) Internal phase or Dispersed phase (Discontinuous phase): It is the component present in small proportion and is just like a solute in a solution. For instance in the colloidal solution of silver in water (silver acts as a dispersed phase)
(ii) External phase or Dispersion medium (continuous phase): It is usually component present in excess and is just like a solvent in a solution. For instance, in the colloidal solution of silver in water. Hear water act as a medium of dispersion.
(2) Classification of colloids : The colloids are classified on the basis of the following criteria
(i) The classification based on physical state of the dispersed phase and dispersion medium : Depending upon the physical state of the dispersed phase and dispersion medium whether these are solids, liquids or gases, eight types of colloidal systems are possible.
Table: Different types of colloidal systems
|
Dispersed phase
|
Dispersion Medium
|
Colloidal System
|
Examples
|
|
Liquid
|
Gas
|
Aerosol of liquids
|
Fogs, clouds, mists, fine insecticide sprays
|
|
Solid
|
Gas
|
Aerosol of solids
|
Smoke, volcanic dust, haze
|
|
Gas
|
Liquid
|
Foam or froth
|
Soap lather. Lemonade froth, foam, whipped cream, soda water
|
|
Liquid
|
Liquid
|
Emulsions
|
Milk, emulsified oils, medicines
|
|
Solid
|
Liquid
|
Sols
|
Most paints, starch in water, proteins, gold sol, arsenic sulphide sol, ink
|
|
Gas
|
Solid
|
Solid foam
|
Pumice stone, styrene rubber, foam rubber
|
|
Liquid
|
Solid
|
Gels
|
Cheese, butter, boot polish, jelly, curd
|
|
Solid
|
Solid
|
Solid sols (coloured glass)
|
Ruby glass, some gem stones and alloys
|
(ii) The Classification based on Nature of the interaction among dispersed phase and the dispersion medium: Depending upon the nature of interactions between dispersed phase and the dispersion medium, the colloidal solutions can be classified into two types as (a) Lyophilic and (b) Lyophobic sols.
(a) Lyophilic colloids (water loving) : "The colloidal solutions in which the particles of the dispersed phase have a great affinity for the dispersion medium, are known as lyophilic collodis."
(b) Lyophobic colloids (water hateing) : "The colloidal solutions in which there is no affinity between particles of the dispersed phase and the dispersion medium are called lyophobic colloids."
Distinction between lyophilic and lyophobic sols
|
Property
|
Lyophilic sols (suspensoid)
|
Lyophobic sols (Emulsoid)
|
|
Surface tension
|
Lower than that of the medium
|
Same as that of the medium
|
|
Viscosity
|
Much higher than that of the medium
|
Same as that of the medium
|
|
Reversibility
|
Reversible
|
Irreversible
|
|
Stability
|
More stable
|
Less stable
|
|
Visibility
|
Particles can't be detected even under ultramicroscope
|
Particles can be detected under ultramicroscope.
|
|
Migration
|
Particles may migrate in either direction or do not migrate in an electric field because do not carry any charge.
|
Particles migrate either towards cathode or anode in an electric field because they carry charge.
|
|
Action of electrolyte
|
Addition of smaller quantity of electrolyte has little effect
|
Coagulation takes place
|
|
Hydration
|
Extensive hydration takes place
|
No hydration
|
|
Examples
|
Gum, gelatin, starch, proteins, rubber etc.
|
Metals like Ag and Au, hydroxides like Al(OH)3,Fe(OH3metal sulphides like As2S3 etc.
|
(iii) The Classification based on the types of particle of the dispersed phase : Depending upon the type of the particles of the dispersed phase, the colloids are classified as follows.
- (a) Multimolecular colloids When on dissolution, atoms or smaller molecules of substances (having diameter less than 1nm) aggregate together to form particles of colloidal dimensions, the particles thus formed are called multimolecular colloids.
- In this type of sols the dispersed phase consists of aggregates of atoms or molecules with molecular size less than 1 nm.
- For example, sols of gold atoms and sulphur (S8) molecules. In such colloids, the particles are held together by Vander Waal's forces. They have generally lyophilic character.
(b) Macromolecular colloids
- These are the substances having big size molecules (called macromolecules) which on dissolution form size in the colloidal range. These types of substances are called as macromolecular colloids.
- These macromolecules creating the dispersed phase are generally polymers having very high molecular masses.
- Naturally occurring macromolecules are cellulose, enzymes proteins, starch, , gelatin etc. The artificial macromolecules are the synthetic polymers like polythene, nylon, plastics, polystyrene etc.
- They have generally lyophobic character.
(c) Associated colloids
- These are the substances which on dissolved in a medium behave as normal electrolytes at low concentration however behave, as colloidal particles at higher concentration due to the formation of the aggregated particles. Aggregates particles thus formed are termed as micelles.
- Their molecules contain both lyophilic and lyophobic groups.
Micelles
- Micelles are cluster or the aggregated particles formed by association of colloid in solution.
- The common examples of micelles are soaps and detergents.
- Formation of the micelles takes place over the particular temperature called Kraft temperature (Tk) and above a particular concentration called critical micellization concentration (CMC).
- They are capable of forming ions.
- Micelles can contain as many as 100 molecules or more than that.
- For example sodium stearate (C17H35COONa) is a typical example of such type of molecules.
- When the sodium stearate is dissolved in water, it gives Na+ and the C17H35COO- ions.
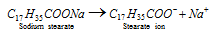
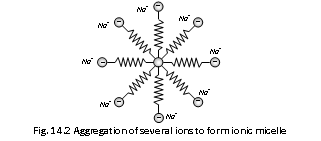
Stearate ions associate to form the ionic micelles of colloidal size.
- It has long hydrocarbon part of C17H35 radical. Which is lyophobic and COO- part which is lyophilic.
- In the figure, the chain corresponds to stearate ion, (C17H35COO-). When the concentration of the solution is below from its CMC (10-3 mol L-1), it behaves as normal electrolyte. Although above this concentration it is aggregated to behave as micelles.
- The main function of a soap is to reduce oily and greasy dirt to colloidal particles (an emulsion). Soap hence, is called as emulsifying agents.
- Some examples of micelles are sodium palmitate (C17H35COONa), Sodium lauryl sulphate [CH3(CH2)11SO3O-Na+], Cetyl trimethyl ammonium bromide CH3(CH2)15(CH2)3N+Br- etc.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Phases of the colloids and their classification questions? Phases of the colloids and their classification topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Phases of the colloids and their classification related problems. We provide step by step Phases of the colloids and their classification question's answers with 100% plagiarism free content. We prepare quality content and notes for Phases of the colloids and their classification topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours