The Origin of charge on the colloidal particles
The origin of the charge on the sol particles in most cases is due to the preferential adsorption of either positive or negative ions on their surface. The solution particles acquire the electrical charge in any one or more of the following given ways.
(1) Due to the dissociation of the surface molecules : Some colloidal particles develope electrical charge due to the dissociation / ionization of the surface molecules. Charge on the colloidal particles can be balanced by the oppositely charged ions in the sol. For instance, an aqueous solution of soap (sodium palmitate) which dissociates into ions as,
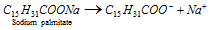
The cations (Na+) pass into the solution while the anions (C15H31COO) have a tendency to form aggregates due to weak attractive forces present in the hydrocarbon chains.
(2) Due to frictional electrification
(i) It is supposed that the frictional electrification due to the rubbing of the dispersed phase particles with that of dispersion medium results in some charge on the colloidal particles.
(ii) The dispersion medium must also get some charge, due to the friction. AS it does not carry any charge, theory does not appear to be correct.
(3) Due to selective adsorption of ions
(i) The particles having the dispersed phase adsorb only those ions preferentially which are common with their own lattice ions.
(ii) For example, when a small quantity of silver nitrate (AgNO3) solution is added to a large quantity of potassium iodide KIsolution, colloidal particles of the silver iodide adsorb I- from the solution to become negatively charged, (at this stage KI is in excess, and I-being common to AgI)
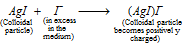
But, when a small quantity of potassium iodide (KI) solution is added to a large quantity of silver nitrate solution (AgNO3); the colloidal silver iodide particles adsorb Ag+ from the solution to become positively charged, (at this stage AgNO3 is in excess and Ag+ is common to AgI),
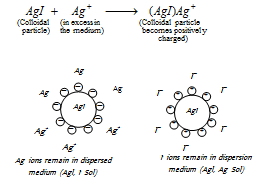
(iii) Depending upon the nature of charge on the particles of the dispersed phase, the colloidal solutions can be classified into positively charged and negatively charged colloids. Some of the typical examples of it are as follows
|
(a) Negatively charged
colloids
|
(b) Positively charged
colloids
|
|
As2S3, CdS
- Metal dispersions : Ag, Au, Pt
- Acid dyes : Eosin, congo red
- Sols of starch, gums, gold,
gelatin etc.
|
Al(OH)3, Fe(OH)3
- Basic dyes : Methylene blue
|
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Origin of charge on colloidal particles questions? Origin of charge on colloidal particles topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Origin of charge on colloidal particles related problems. We provide step by step Origin of charge on colloidal particles question's answers with 100% plagiarism free content. We prepare quality content and notes for Origin of charge on colloidal particles topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours