Nuclear Binding Energy and the Mass defect: It is observed that the atomic mass of all the nuclei (excluding hydrogen) is different from the sum of the masses of protons and neutrons. The difference is called as mass defect.
Mass defect = Total mass of nucleons - obs. atomic mass
The mass defect can be converted into energy. This energy is called as binding energy. This is the energy needed to break the nucleus into its constituents (which are p and n).
Binding energy = Mass defect * 931 MeV
The stability of nucleus is explained on the value of the binding energy per nucleon and not on basis of the total binding energy. Binding energy per nucleon is highest (8.7 MeV) in the case of iron (56). The value of the binding energy per nucleon can be increased either by fusion of the lighter nuclei or by the fission of heavier nuclei.
The value of binding energy predicts relative stability of the different isotopes of the element. If the value of binding energy is negative in nature, then the product nucleus or nuclei will be much less stable than the reactant nucleus. Therefore the relative stability of the different isotopes of an element can be predicted by values of binding energy for each of the successive addition of one neutron to nucleus.
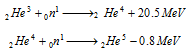
Hence, 2He4 is more stable than 2He3 and 2He5.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Nuclear Binding Energy and Mass Defect questions? Nuclear Binding Energy and Mass Defect topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Nuclear Binding Energy and Mass Defect related problems. We provide step by step Nuclear Binding Energy and Mass Defect question's answers with 100% plagiarism free content. We prepare quality content and notes for Nuclear Binding Energy and Mass Defect topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours