IUPAC system : In order to rationalise the system of naming, an International Congress of Chemists was held in Geneva in 1892. They adopted certain uniform rules for naming the compounds.
The system of nomenclature was named as Geneva system. Since then the system of naming has been improved from time to time by the International Union of Pure and Applied Chemistry and the new system is called IUPAC system of naming. This system of nomenclature was first introduced in 1947 and was modified from time to time. The most exhaustic rules for nomenclature were first published in 1979 and later revised and updated in 1993. The rules discussed in the present chapter are based on guide books published by IUPAC in 1979 (Nomenclature of Organic Chemistry by J. Rigandy and S.P. Klesney) and 1993 (A Guide to IUPAC Nomenclature for Organic Chemistry by R. Panico, W.H. Powell and J.C. Richer). With the help of this system, an organic compound having any number of carbon atoms can be easily named.
IUPAC System of Naming Organic Compounds : In the IUPAC system, the name of an organic compound consist of three parts : (i) Word root (ii) Suffix (iii) Prefix
(i) Word root : The word root denotes the number of carbon atoms present in the chain.
Table:
Chain length
|
Word root
|
Chain length
|
Word root
|
|
C1
|
Meth-
|
C11
|
Undec-
|
|
C2
|
Eth-
|
C12
|
Dodec-
|
|
C3
|
Prop-
|
C13
|
Tridec-
|
|
C4
|
But-
|
C14
|
Tetradec-
|
|
C5
|
Pent-
|
C15
|
Pentadec-
|
|
C6
|
Hex-
|
C16
|
Hexadec-
|
|
C7
|
Hept-
|
C17
|
Heptadec-
|
|
C8
|
Oct-
|
C18
|
Octadec-
|
|
C9
|
Non-
|
C19
|
Nonadec-
|
|
C10
|
Dec-
|
C20
|
Eicos
|
(ii) Suffix : The word root is related to the suffix which may be primary or secondary or both.
(a) Primary suffix : A primary suffix is added to the word root to indicate whether the carbon chain is saturated or unsaturated.
Table:
Type of carbon chain
|
Primary suffix
|
General name
|
|
Saturated (C - C)
|
-ane
|
Alkane
|
|
Unsaturated (C = C)
|
-ene
|
Alkene
|
|
Unsaturated (C Ξ C)
|
-yne
|
Alkyne
|
If the parent chain has two, three or more triple or double bonds, then the numerical prefixes such as di (for two), tri (for three), tetra (for four), etc. are added to the primary suffix.
(b) Secondary suffix : A secondary suffix is then added to the word root after the primary suffix to indicate the functional group present in the organic compound.
Table:
Class of org. compound
|
Functional group
|
Secondary suffix
|
|
Alcohols
|
-OH
|
-ol
|
|
Aldehydes
|
- CHO
|
-al
|
|
Ketones
|
> C = O
|
-one
|
|
Carboxylic acids
|
- COOH
|
-oic acid
|
|
Esters
|
- COOR
|
alkyl.... oate
|
|
Acid chlorides
|
- COCl
|
-oyl chloride
|
|
Acid amides
|
- CONH2
|
-amide
|
|
Nitriles
|
- C Ξ N
|
-nitrile
|
|
Amines
|
- NH2
|
-amine
|
|
Thiol
|
- SH
|
thiol
|
It may be noted that while adding the secondary suffix to the primary suffix, the terminal 'e' of the primary suffix (i.e. ane, ene and yne) is droped if the secondary suffix begins with a vowel but is retained if the secondary suffix begins with a consonant. As given
Organic compound
|
CH3CH2OH
|
CH3CH2CN
|
|
Word root
|
Eth
|
Prop
|
|
Primary suffix
|
an (e)*
|
ane
|
|
Secondary suffix
|
ol
|
nitrile
|
|
IUPAC name
|
Ethanol
|
Propanenitrile
|
The terminal 'e' from the primary suffix has been dropped because the secondary suffix i.e. 'ol' begins with a vowel 'o'.
(iii) Prefix : There are many groups which are not regarded as functional groups in the IUPAC name of the substance. These are related as substituents or side chains. These are represented as prefixes and are placed before the word root while naming a particular compound. These may be :
(a) Alkyl groups : These groups contain one hydrogen atom less than the alkane. These are named by substituting the suffix ane of the name of the corresponding alkane by yl. i.e. alkane - ane + yl = alkyl.
For example,
CH4 : Methane becomes
CH3- : Methyl
CH3 CH3 : Ethane becomes CH3 CH2- : Ethyl
CH3 CH2 CH3 : Propane becomes CH3 CH2 CH2 : Propyl etc.
(b) Functional groups not regarded as principal functional groups : If a compound contains more than one functional group, then one of the functional group is regarded as principal functional group and other is treated as secondary suffix. The other functional groups are regarded as substituents and are indicated by prefixes.
Table:
Substituent
|
Prefix
|
|
-F
|
Fluoro
|
|
-Cl
|
Chloro
|
|
-Br
|
Bromo
|
|
-I
|
Iodo
|
|
- NO
|
Nitroso
|
|
- N = N -
|
Diazo
|
|
-OCH3
|
Methoxy
|
|
-OC2H5
|
Ethoxy
|
|
- NO2
|
Nitro
|
|
- NH2
|
Amino
|
|
-OH
|
Hydroxo
|
Thus, a complete IUPAC name of an organic compound may be represented as:
Prefix + word root + Primary suffix + Secondary suffix
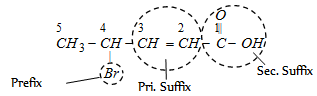
Word root : Pent (five C - C - C - C - C)
Primary suffix : ene (double bond at C - 2)
Secondary suffix : oic acid (- COOH group)
Prifix : Bromo (- Br group at C - 4)
IUPAC name : Bromo + pent + ene + oic acid or 4-Bromopent -2-en-1-oic acid
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Nomenclature of organic compounds-IUPAC System questions? Nomenclature of organic compounds-IUPAC System topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Nomenclature of organic compounds-IUPAC System related problems. We provide step by step Nomenclature of organic compounds-IUPAC System question's answers with 100% plagiarism free content. We prepare quality content and notes for Nomenclature of organic compounds-IUPAC System topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours