Molecular orbital theory or MOT
The molecular orbital theory was given by the3 scientists Hund and Mulliken in the year 1932.
The basic ideas of this theory are,
(1) When the two atomic orbitals combine or overlap with each other, they lose their identity and form the new orbitals. The new orbitals so formed are called as molecular orbitals.
(2) The molecular orbitals are the energy states of the molecule in which the electrons of the molecule are filled just as the atomic orbitals are the energy states of an atom in which the electrons of atom are filled.
(3) In the terms of probability distribution, a molecular orbital provides the electron probability distribution around the group of nuclei just as an atomic orbital provides the electron probability distribution around single nucleus.
(4) Only those atomic orbitals can combine to form the molecular orbitals which posses comparable energies and proper orientation.
(5) Number of molecular orbitals formed is equal to number of the combining atomic orbitals.
(6) When the two atomic orbitals combine, they form two new orbitals called as the bonding molecular orbital and the antibonding molecular orbital.
(7) Bonding molecular orbital has lower energy and hence the greater stability than corresponding antibonding molecular orbital.
(8) Bonding molecular orbitals are represented as σ, ∏ etc, whereas corresponding antibonding molecular orbitals are represented as σ+, ∏+ etc.
(9) The shapes of molecular orbitals formed depend upon type of combining atomic orbitals.
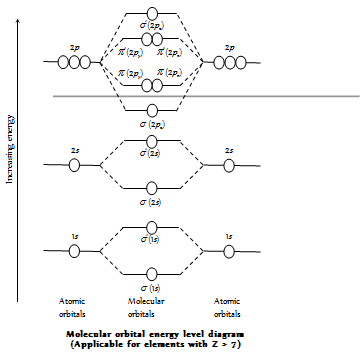
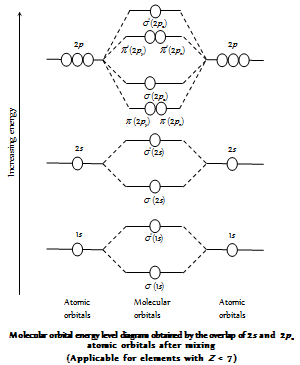
(10) The filling of the molecular orbitals in a molecule takes place in accordance with the Pauli's exclusion principle, Aufbau principle, and the Hund's rule. In the general order of the increasing energy among the molecular orbitals formed by the elements of the second period and hydrogen and their general electronic configurations can be given as follows.
(11) The electrons are filled in the increasing energy of MO which is in the order given below
(a) 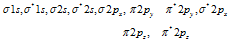
(b) 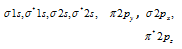
Increasing energy (for the electrons ≤14)
(12) The number of bonds between the two atoms is called as the bond order and is given as follows
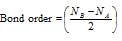
Here NB the number of electrons in bonding MO.
NA the number of electrons in the antibonding MO.
For a stable molecule/ion, NB > NA
(13) 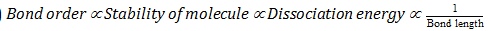
(14) If all electrons in the molecule are paired then the substance is a diamagnetic on the other hand if there are unpaired electrons in molecule, then the substance is paramagnetic in nature. More the number of unpaired electron in the molecule greater will be the paramagnetism of the substance.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Molecular Orbital Theory or MOT questions? Molecular Orbital Theory or MOT topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Molecular Orbital Theory or MOT related problems. We provide step by step Molecular Orbital Theory or MOT question's answers with 100% plagiarism free content. We prepare quality content and notes for Molecular Orbital Theory or MOT topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours