The mole concept
One mole of any of the substance contains a fixed number (6.022 * 1023) of any type of particles (atoms or molecules or ions) and contains a mass equal to the atomic or molecular weight, in grams. Therefore it is correct to refer to a mole of helium, a mole of electrons, or a mole of Na+, meaning respectively Avogadro's number of electrons, atoms, or ions.
Number of moles 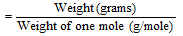
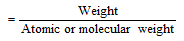
The percentage composition & Molecular formula
(1) The percentage composition of the compound
Percentage composition of the compound is the relative mass of each of the constituent element in the 100 parts of it. If molecular mass of the compound is M and B is the mass of an element in the molecule, then

(2) Determination of empirical formula : The empirical formula of a molecule is determined using the % of elements present in it. The below stated method is adopted.
|
Element
|
% Relative no. of atoms = %/at. wt.
|
Simplest Ratio
|
Empirical Formula
|
Relative no. of atoms : Divide the percentage of each element present in compound by its at weight. This provides the relative number of atoms of element in molecule.
Simplest ratio: Fit is used to find out lowest value of relative number of atoms and then divide each value of relative number of atoms by this value to estimate simplest ratio of elements.
If the simplest ratio attained is not complete integers, then multiply them by a common factor to get the integer values of simplest ratio.
Empirical formula: Write all constituent atoms with their respective number of atoms derived in the simplest ratio. This gives the empirical formula of compound.
Molecular formula: Molecular formula = n * empirical formula where 'n' is the whole number obtained as follows
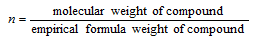
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Mole concept questions? Mole concept topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Mole concept related problems. We provide step by step Mole concept question's answers with 100% plagiarism free content. We prepare quality content and notes for Mole concept topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours