Methane : Known as marsh gas.
(i) Industrial method of preparation : Mathane gas is obtained on a large scale from natural gas by liquefaction. It can also be obtained by the application of following methods,
(a) From carbon monoxide : A mixture of carbonmonoxide and hydrogen is passed over a catalyst containing nickel and carbon at 250oC when methane is formed.
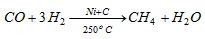
(b) Bacterial reduction of cellulose material present in sewage water : This method is being used in England for production of methane.
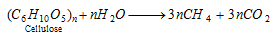
(c) Synthesis : q By striking an electric field between carbon electrodes in an atmosphere of hydrogen at 1200oC, methane is formed.
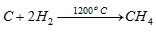
By passing a mixture of hydrogen sulphide and carbon disulphide vapour through red hot copper, methane is created.
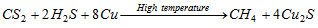
(ii) Physical properties
(a) It is an odourless, colourless, tasteless and non-poisonous gas.
(b) It is lighter than air. Its density at NTP is 0.71 g/L.
(c) It is slightly soluble in water but is quite soluble in ether, alcohol and acetone.
(d) Its melting point is -182oC and boiling point is -161.5oC.
(iii) Uses
(a) In the development of compounds like formaldehyde, methyl chloride, methyl alcohol, chloroform, carbon tetrachloride, etc.
(b) In the preparation of hydrogen, used for creating ammonia.
(c) In the manufacturing of carbon black which is used for making printing ink, black paints and as a filler in rubber vulcanisation.
(d) As a fuel and illuminant.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Methane questions? Methane topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Methane related problems. We provide step by step Methane question's answers with 100% plagiarism free content. We prepare quality content and notes for Methane topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours