Mercury:
The extraction and occurrence of mercury:
The cinnabar (i.e., HgS) is the only vital ore of Hg. This ore is concentrated through froth floatation technique and this is extracted from this ore by heating it in air at the temperature of 773-873 K (i.e., auto reduction).
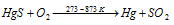
The mercury vapors so obtained are condensed to provide liquid metal. Hg so obtained comprises impurities of Sn Zn, and Pb. These can be eliminated by treating the impure metal with dilute HNO3, mercurous nitrate, Hg2 (NO3)2 as a result made react with metals existed as impurities forming their nitrates that pass into solution leaving behind pure mercury. However, it is finest purified by distillation beneath decreased pressure.
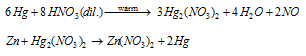
Identical reaction is offered through Pb and Sn.
Properties of mercury:
Hg is very less reactive than Zn. This is liquid at room temperature and has pretty low thermal and electrical conductivity. Mercury builds up dimeric mercury (I) ions, Hg22+ in which two atoms are bonded by the covalent bond. This is steadily oxidized onto HgO at as regards its boiling point. Hg does not react with dilute HCl or dil.H2SO4 however reacts with hot concentrated H2SO4 to make HgSO4, it reacts with warm dilute and concentrated HNO3 both sprouting NO and NO2 correspondingly.
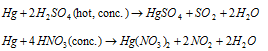
Hg does not react with steam or water and hence cannot make any hydroxide.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Mercury questions? Mercury topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Mercury related problems. We provide step by step Mercury question's answers with 100% plagiarism free content. We prepare quality content and notes for Mercury topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours