Mechanism of the reaction
(1) Reaction making use of first order consecutive reactions
(i) In this kind of reactions, the reactions from the stable intermediate compound before they are finally converted into products.
(ii) For instance, reactants (R) are first converted to intermediate (I) which is then converted to product (P) as
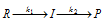
Thus, the reaction takes place in two steps, both of which are of the first order that is
Step I : 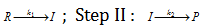
This means that I is obtained by step I and consumed by step II. In the above given reactions, each stage will have its own rate and rate constant reactant concentration will always decrease and product concentration will always increase as which is shown in figure
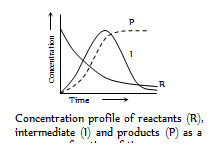
(2) Reaction involving slow step : When a reaction occurs by a sequence of steps and one of the step is quite slow, then rate determining step is slow step. For instance in the reaction
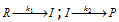 ; , if k1<<k2 then I is converted into the products as soon as it is formed, we get the conclusion as follows
; , if k1<<k2 then I is converted into the products as soon as it is formed, we get the conclusion as follows
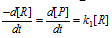
(3) Parallel reactions : In these type of reactions the reactants are more reactive, which can have different orders of the reactions taking place simultaneously. For example, in a system containing NO2 and SO2, NO2 is consumed in the following two reactions,
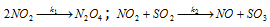
The rate at which the NO2 disappears will be sum of the rates of the two reactions i.e.,
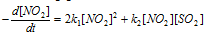
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Mechanism of the reaction questions? Mechanism of the reaction topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Mechanism of the reaction related problems. We provide step by step Mechanism of the reaction question's answers with 100% plagiarism free content. We prepare quality content and notes for Mechanism of the reaction topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours