Mechanism of rusting of iron : Electrochemical theory of rusting.
The overall rusting involves the following steps,
(i) Oxidation occurs at the anodes of each electrochemical cell. Therefore, at each anode neutral iron atoms are oxidised to ferrous ions.
At anode : 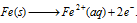
Thus, the metal atoms in the lattice pass into the solution as ions, leaving electrons on the metal itself. These electrons move towards the cathode region through the metal.
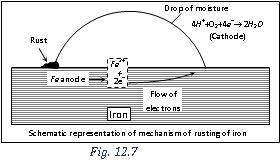
(ii) At the cathodes of each cell, electrons are taken up bythe hydrogen ions (reduction occurs). The H+ ions are obtained either from water or from acidic substances (e.g. CO2 in water)
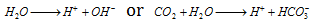
At cathode : 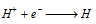
The hydrogen atoms on the iron surface reduce dissolved oxygen.
Therefore, the overall reaction at cathode of different electrochemical cells may be written as,
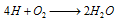
(iii) The overall redox reaction may be written by multiplying reaction at anode by 2 and adding reaction at cathode to equalise number of electrons lost and gained i.e.
Oxi. half reaction : 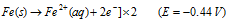
Red. half reaction : 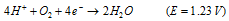
Overall cell reaction : 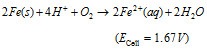
The ferrous ions are oxidised further by atmospheric oxygen to form rust.
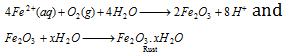
It may be noted that salt water accelerates corrosion. This is majorly because of the fact that salt water increases the electrical conduction of electrolyte solution formed on the metal surface. Thus, rusting becomes more serious problem where the salt water is present.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Mechanism of rusting of iron questions? Mechanism of rusting of iron topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Mechanism of rusting of iron related problems. We provide step by step Mechanism of rusting of iron question's answers with 100% plagiarism free content. We prepare quality content and notes for Mechanism of rusting of iron topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours