Photochemical combination of H2 and Cl2 : A mixture of H2 and Cl2 on exposure to light give rise to the formation of HCl, showing a chain reaction and thereby producing 106 to 108 molecules of HCl per photon absorbed.
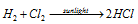
The mechanism leading to very high yield of HCl as a result of chemical change can be as follows. Molecule of the chlorine absorbs radiant energy to form the excited molecule which decomposes to chlorine free radicals (Cl) to give chain initiation step.
Light absorption step: 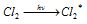 ........(i)
........(i)
Chain initiation step: 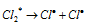 ........(ii)
........(ii)
The chlorine free radical then combines with H2 molecule to form HCl and 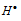 free radical. The
free radical. The 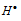 free radical so formed again combines with another Cl2 molecule to give HCl and
free radical so formed again combines with another Cl2 molecule to give HCl and 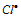 free radical back resulting into chain propagation step.
free radical back resulting into chain propagation step.
Chain propagation step : 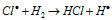 ........(iii)
........(iii)
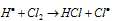
The combination of two 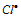 free radicals leads to chain terminating step.
free radicals leads to chain terminating step.
Chain terminating step : 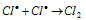 ........(iv)
........(iv)
(ii) Photochemical combination of H2 and Br2 : The combination of H2 H2 and Br2 to form HBr in presence of light is also an example of chain reaction like photochemical combination of H2 and Cl2. Here two Br2 molecules absorb photon, though, insteed of the chain reaction one molecule of HBr is formed for each 100 photon absorbed by 100 molecules of Br2.
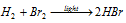
Mechanism
Light absorption step : 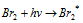 ........(a)
........(a)
Chain initiation step : 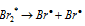 ........(b)
........(b)
Chain propagation step : 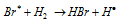 ........(c)
........(c)
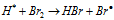 ........(d)
........(d)
Chain termination step : 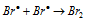 ........(e)
........(e)
The lower values of HBr formation per photon of light absorbed has been attributed to the fact that step (III) is highly endothermic and thus before step (III) can take place most of the bromine free radicals recombine as per step (V) to give Br2 molecule and thus providing less feasibility for step (IV) i.e. steps regenerating free radicals. The decomposition of HBr also increases with increase in temperature.
(3) Quantum yield (or quantum efficiency) : The quantum efficiency or yield 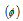 of a photochemical reaction may be expressed as,
of a photochemical reaction may be expressed as,

Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Mechanism of photochemical reactions questions? Mechanism of photochemical reactions topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Mechanism of photochemical reactions related problems. We provide step by step Mechanism of photochemical reactions question's answers with 100% plagiarism free content. We prepare quality content and notes for Mechanism of photochemical reactions topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours