The mass action law and the Equilibrium constant
On the basis of observations of various equilibrium reactions, the two Norwegian chemists which were Goldberg and Waage suggested (in the year 1864) a quantitative relationship between the rates of reactions and the concentration of the reacting substances. This relationship is called as law of mass action. It can be stated as follows
The Rate of chemical is directly proportional to the product of the molar concentrations of reactants at the constant temperature at any given time.
The molar concentration that is the number of moles per liter is also known active mass. It is expressed by enclosing symbols of formulae of the substance in the square brackets. For instance, molar concentration of A is expressed as [A].
Consider the simple reversible reaction which is given as follows
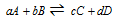 (At a certain temperature)
(At a certain temperature)
According to law of mass action
Rate of forward reaction 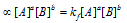
Rate of backward reaction 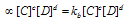
At equilibrium ,
The rate of forward reaction = The Rate of backward reaction
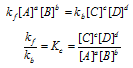
Here, is called as equilibrium constant.
In terms of partial pressures, equilibrium constant is denoted by Kp and
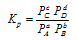
In terms of mole fraction, equilibrium constant is denoted by Kx and
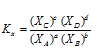
Relation between Kp, Kc and Kx
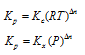
Dn = number of moles of gaseous products - number of moles of gaseous reactants in chemical equation.
As a rule, the concentration of pure solids and pure liquids are not included when writing an equilibrium equation.
|
Value of Dn
|
Relation between Kp and Kc
|
Units of Kp
|
Units of Kc
|
|
0
|
Kp = Kc
|
No unit
|
No unit
|
|
>0
|
Kp > Kc
|
(atm)Dn
|
(mole l-1)Dn
|
|
<0
|
Kp < Kc
|
(atm)Dn
|
(mole l-1)Dn
|
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Mass action law and the Equilibrium constant questions? Mass action law and the Equilibrium constant topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Mass action law and the Equilibrium constant related problems. We provide step by step Mass action law and the Equilibrium constant question's answers with 100% plagiarism free content. We prepare quality content and notes for Mass action law and the Equilibrium constant topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours