Liquefaction of gases
(1) A gas can be liquefied by cooling or by application of high pressure or by the combined effect of both. The first successful attempt for the liquefying of gases was made by Faraday.
(2) Theases for which the intermolecular forces of attraction are quite small such as H2, N2, Ar and O2, have the low values of Tc and cannot be liquefied by application of pressure are termed as permanent gases while the gases for which intermolecular forces of attraction are large, like polar molecules NH3, SO2 and H2O have quite high values of Tc and can be liquefied much easily.
(3) The methods of liquefaction of the gases : The modern methods of cooling gas to or below their Tc and thus of liquefaction of gases are done by the Linde's method and Claude's method.
(i) Linde's method : This process is based upon the Joule-Thomson effect which states that When the gas is permitted to expand adiabatically from a region of high pressure to the region of extremely low pressure, it is accompanied by the process of cooling."
(ii) Claude's method : This process is based on the principle that when the gas expands adiabatically against an external pressure (as a piston in the engine), it perform some external work. Since the work is done by the molecules at cost of their kinetic energy, the temperature of the gas falls resulting in cooling.
(iii) By adiabatic demagnetisation.
(4) Uses of liquefied gases : The liquefied and gases compressed under the high pressure are of great importance in the industries.
(i) The liquid ammonia and liquid sulphur dioxide are taken in use as refrigerants.
(ii) Liquid carbon dioxide finds use in the soda fountains and several other uses.
(iii) The liquid chlorine is used for the bleaching and disinfectant processes.
(iv) The liquid air is an important source of the oxygen in rockets and jet-propelled planes and the bombs as well.
(v) Compressed oxygen is taken in use for welding purposes.
(vi) Compressed helium is used in the airships as a source of fuel.
(5) Joule-Thomson effect : When thea real gas is allowed to expand adiabatically through a porous plug or a fine hole into a region of low pressure, it is accompanied by cooling (except for hydrogen and helium which get warmed up).
The cooling takes place because some of the work is done to overcome the intermolecular forces of the attraction. As a result of which, the internal energy decreases and so does temperature.
Ideal gases do not show any kind of cooling or heating because there are no intermolecular forces of attraction that is they do not show Joule-Thomson effect.
During the Joule-Thomson effect, enthalpy of system remains constant.
Joule-Thomson coefficient. 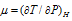 .
.
For cooling, µ = +ve (because dT and dP will be -ve)
For heating µ = -ve (because dT = +ve, dP = -ve).
For no heating or cooling µ = 0 (because dT = 0.
(6) Inversion temperature : It is the temperature at which gas shows neither cooling effect nor heating effect that is Joule-Thomson coefficient µ = 0. Below this temperature, it shows cooling effect and above this temperature, it shows the heating effect.
Any gas like H2, He etc, whose inversion temperature is low would show heating effect at room temperature. However, if these gases are just cooled below inversion temperature and then subjected to Joule-Thomson effect, they will also undergo cooling.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Liquefaction of gases questions? Liquefaction of gases topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Liquefaction of gases related problems. We provide step by step Liquefaction of gases question's answers with 100% plagiarism free content. We prepare quality content and notes for Liquefaction of gases topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours