The Limiting reagent or reactant
In number of situations, an excess of one or more substance is available for the chemical reaction. Some out of these excess substances will therefore be left over when the reaction is terminates; the reaction stops immediately as soon as one of the reactant is totally consumed.
The substance which is totally consumed in a reaction is called the limiting reagent because it determines or limits the amount of the product. The other reactant present in excess is called as the excess reagents.
Let us take a chemical reaction which is initiated by passing the spark through a reaction vessel containing 10 mole of H2 and 7 mole of O2.
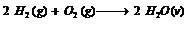
|
Moles before reaction
|
10
|
7
|
0
|
|
Moles after reaction
|
0
|
2
|
10
|
The reaction stops only after the consumption of 5 moles of O2 as no further amount of H2 is left to react with the unreacted O2. Therefore H2 is a limiting reagent in this reaction.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Limiting reagent or reactant questions? Limiting reagent or reactant topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Limiting reagent or reactant related problems. We provide step by step Limiting reagent or reactant question's answers with 100% plagiarism free content. We prepare quality content and notes for Limiting reagent or reactant topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours