Ligand field theory : According to this theory when the ligands come closer to metal atom or ion, a field is created. This field tends to split the degenerate d-orbitals of the metal atom into different energy levels. The nature and number of ligands determine the extent of splitting. Energy gap between the splitted energy levels determine the magnetic (paramagnetic or diamagnetic) and spectral properties (colour etc) of the complexes .
Stability of co-ordination in solution and Spectrochemical series
The stronger is metal-ligand bond, less will be the dissociation in the solution and hence greater is the stability of coordination compounds.
The instability constant for complex ion can be given by [Cu(NH3)4]2+ i.e.
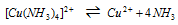 , is given by the expression;
, is given by the expression; 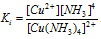
The stability constant of the above complex that is
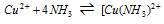 is given as under ;
is given as under ; 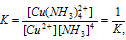
Greater is the stability constant, stronger is the metal - ligand bond
The factors which affect the stability of complex ion
(1) Nature of central metal ion : The higher the charge density on the central metal ion the greater is the stability of the complex
For example, the stability constant of [Fe(CN)6]3- is much greater than the stability constant of [Fe(CN)6]4-.
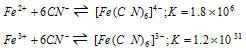
Effective atomic number (EAN) or Sidgwick theory : In order to the stability of the complexes sidgwick proposed effective atomic number. EAN usually coincides with the atomic number of next noble gas in some cases. EAN can be calculated by the following relation :
EAN = Atomic no. of the metal - e- lost in ion formation +No. of e- gained from the donor atom of the ligands.
EAN = Atomic number - Oxidation number + co-ordination no. ´2
Table
|
Complex
|
Metal oxidation state
|
At. No. of metal
|
Coordination number
|
Effective atomic number
|
|
K4[Fe(CN)6]
|
+ 2
|
26
|
6
|
(26 - 2) + (6 ´ 2) = 36 [Kr]
|
|
[Cu(NH3)4]SO4
|
+ 2
|
29
|
4
|
(29 - 2) + (4 ´ 2) = 35
|
|
[Co(CH3)6]Cl3
|
+ 3
|
27
|
6
|
(27 - 3) + (6 ´ 2) = 36 [Kr]
|
|
Ni(CO)4
|
0
|
28
|
4
|
(28 - 0) + (4 ´ 2) = 36 [Kr]
|
|
K2[Ni(CN)4]
|
+ 2
|
28
|
4
|
(28 - 2) + (4 ´ 2) = 34
|
|
K3[Cr(C2O4)3]
|
+ 3
|
24
|
6
|
(24 - 3) + (6 ´ 2) = 33
|
|
K3[Fe(CN)6]
|
+ 3
|
26
|
6
|
(26 - 3) + (6 ´ 2) = 35
|
|
[Ag(NH3]2Cl
|
+ 1
|
47
|
2
|
(47 - 1) + (2 ´ 2) = 50
|
(2) Nature of ligand : Greater the base strength is the ease with which it can donate its lone pair of electrons and therefore, greater is the stability of the complex formed by it.
The example of it is : [Cu(NH3)4]2+; K = 4.5 * 1011; [Cu(CN)4]2-; K = 2.0 * 1027
(3) Presence of chelate ring : Chelating ligands form more stable complex as compared to monodentate ligands. The example of it is :
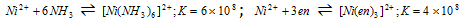
The Spectro chemical series : The ligands can be arranged in increasing order of their strength (ability to cause crystal field splitting) and the series so obtained is called as spectro chemical series.
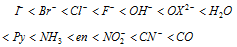
Ligands arranged left to NH3 are generally regarded as weaker ligands which can not cause forcible pairing of electrons within 3d level and thus form outer orbital octahedral complexes.
Conversely NH3 and all ligands lying right to it are stronger ligands which form inner orbital octahedral complexes after forcible pairing of electrons within 3d level.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Ligand Field Theory questions? Ligand Field Theory topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Ligand Field Theory related problems. We provide step by step Ligand Field Theory question's answers with 100% plagiarism free content. We prepare quality content and notes for Ligand Field Theory topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours