Lewis concept: This concept was proposed by the scientist named as G.N. Lewis, in the year 1939. In accordance to this concept, a base is defined as the substance which can furnish the pair of electrons to form a coordinate bond whereas an acid is the substance which can accept the pair of electrons. The acid is also called as electron pair acceptor or the electrophile while the base is electron pair donor or the nucleophile.
A easy example of an acid-base is the reaction of a proton with hydroxyl ion, 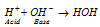
Lewis concept is more general than the Bronsted Lowry concept. All the Bronsted bases are also Lewis bases but all the Bronsted acids are not Lewis acids. [Such as HCl, H2SO4 as they are not capable of accepting a pair of electrons]
(i) Types of Lewis acids: In accordance to Lewis concept, the following species can act as Lewis acids.
(a) The Molecules in which the central atom posses incomplete octet such as BF3, BCl3, AlCl3, BeCl2 etc.
(b) All the cations are likely to act as Lewis acids since they are deficient in electrons.
(c) Molecules in which the central atom has empty d-orbitals. For example SiF4, SnCl4, PF5 etc.
(d) The Molecules having the multiple bonds between the atoms of dissimilar electronegativity for example CO2, SO2.
(ii) Types of Lewis bases: The following species can act as Lewis bases.
(a) The Neutral species having at least one lone pair of the electrons 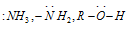
(b) The negatively charged species or the anions
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Lewis concept questions? Lewis concept topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Lewis concept related problems. We provide step by step Lewis concept question's answers with 100% plagiarism free content. We prepare quality content and notes for Lewis concept topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours