Lanthanides : The elements with atomic numbers 58 to 71 that is cerium to lutetium (which come immediately after lanthanum Z = 57) are known as lanthanides or lanthanones or rare earths. These elements include the filling of 4 f-orbitals. Their common electronic configuration can be given as, [Xe]4f1-145d0-106s2. Promethium (Pm), atomic number 61 is the only synthetic (manmade) radioactive lanthanide.
Properties of lanthanides
(i) These are highly dense metals and have high melting points.
(ii) They form alloys easily with other metals especially iron. For example misch metal consists of the rare earth element (94-95%), iron (upto 5%) and traces of C, S, Ca and Al, pyrophoric alloys contain Ce (40-5%), La + neodymium (44%), Fe (4-5%), Al (0-5%) and remaning ratio is of Ca, Si and C. It is used in preparation of ignition devices such as trace bullets and shells and flints for the lighters and cigarette.
(iii) Oxidation state : Most stable oxidation state of lanthanides is +3. Oxidation states + 2 and + 4 also exist but they revert to +3 e.g. Sm2+, Eu2+, Yb2+ lose electron to become +3 and hence are good reducing agents, where as Ce4+, Pr4+, Tb4+ in aqueous solution gain electron to become + 3 and hence are good oxidizing agents. There exists a large gap in the energy of 4 f and 5 d subshells and thus the number of oxidation states is limited.
(iv) Colour : The most of trivalent lanthanide ions are coloured in the solid state and in aqueous solution both. This is because of the partly filled f-orbitals which permit f-f transition. The elements with the xf electrons have a similar colour to those of the (14 - x) electrons.
(v) Magnetic properties : All the lanthanide ions with exception of Lu3+, Yb3+ and Ce 4+ are paramagnetic as they contain unpaired electrons in 4 f orbitals. These elements differ from transition elements in that their magnetic moments do not obey the simple spin only rule 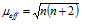 B.M. where n is equal to number of the unpaired electrons. In transition elements, orbital contribution of the electron towards the magnetic moment is generally quenched by interaction with the electric fields of the environment but in case of the lanthanides the 4f-orbitals lie too deep in the atom for such quenching to occur. Thus, magnetic moments of lanthanides are calculated by taking into consideration spin as well as the orbital contributions and a more complex formula
B.M. where n is equal to number of the unpaired electrons. In transition elements, orbital contribution of the electron towards the magnetic moment is generally quenched by interaction with the electric fields of the environment but in case of the lanthanides the 4f-orbitals lie too deep in the atom for such quenching to occur. Thus, magnetic moments of lanthanides are calculated by taking into consideration spin as well as the orbital contributions and a more complex formula
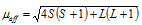 B.M.
B.M.
which includes the orbital quantum number L and spin quantum number S.
(vi) Complex formation: Although the lanthanide ions posses a high charge (+3) yet the size of their ions is very large yielding small charge to size ratio that is low charge density. The consequence of it is that, they have poor tendency to form the complexes. They form complexes mainly with strong chelating agents such as EDTA, β-diketones, oxine etc. No complexes with the Π-bonding ligands are known.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Lanthanides questions? Lanthanides topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Lanthanides related problems. We provide step by step Lanthanides question's answers with 100% plagiarism free content. We prepare quality content and notes for Lanthanides topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours