Langmuir - adsorption isotherms
(i) One of the drawbacks of the Freundlich adsorption isotherm is that it fails at high pressure of the gas. Irving Langmuir in the year 1916 derived a simple adsorption isotherm, on the theoretical considerations based on the kinetic theory of gases. This is termed as Langmuir adsorption isotherm.
(a) The adsorption process takes place on the surface of the solid only till the whole of the surface is completely covered with a unimolecular layer of the adsorbed gas.
(b) The Adsorption consists of the two opposing processes, namely Condensation of gas molecules on the solid surface and Evaporation (desorption) of the gas molecules from the surface back into the gaseous phase.
(c) The rate of condensation depends upon the uncovered (bare) surface of the adsorbent available for condensation. Logically, at the beginning when whole of the surface is uncovered the rate of condensation is very high and as the surface is covered more and more, rate of the condensation progressively decreases. In contrast, the rate of evaporation depends upon the covered surface and hence increases as more and more of surface is covered eventually an equilibrium will be set up at a stage when the rate of condensation becomes equal to the rate of evaporation (adsorption equilibrium).
(d) The rate of condensation also depends upon the pressure of the gas since according the kinetic theory of the gases; the number of molecules striking per unit area is proportional to the pressure.
Mathematically, 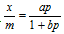 , where a and b are constants and their value depends upon the nature of gas (adsorbate), nature of the solid adsorbent and the temperature. The values of them can be determined from the experimental data.
, where a and b are constants and their value depends upon the nature of gas (adsorbate), nature of the solid adsorbent and the temperature. The values of them can be determined from the experimental data.
Limitation of the Langmuir theory
(a) Langmuir's theory of the unimolecular adsorption is valid only at the low pressures and the high temperatures.
(b) When pressure is increased or the temperature is lowered, additional layers are formed. This has led to modern concept of multilayer adsorption.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Langmuir - adsorption isotherm questions? Langmuir - adsorption isotherm topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Langmuir - adsorption isotherm related problems. We provide step by step Langmuir - adsorption isotherm question's answers with 100% plagiarism free content. We prepare quality content and notes for Langmuir - adsorption isotherm topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours