Kinetic theory of gases
(1) The Kinetic theory was developed by the Bernoulli, Joule, Clausius, Maxwell and the Boltzmann etc and it represents dynamic particle or microscopic model for the different gases since it throws light on behavior of the particles (such as atoms and molecules) which constitute the gases and cannot be seen. Properties of the gases which we studied former are part of the macroscopic model.
(2) Postulates
(i) Every gas comprises of a large number of the small particles called as molecules moving with the very high velocities in all the possible directions.
(ii) The volume of an individual molecule is negligible as compared to total volume of the gas.
(iii) The gaseous molecules are perfectly elastic so that there is no net loss of the kinetic energy due to their collisions.
(iv) The effect of the gravity on the motion of molecules is negligible.
(v) The gaseous molecules are considered as the point masses because they do not have potential energy. So attractive and repulsive forces between the gas molecules are negligible.
(vi) The pressure of the gas is due to continuous bombardment on the walls of the containing vessel.
(vii) At constant temperature average kinetic energy of all the gases is same.
(viii) The average kinetic energy of the gas molecules is directly proportional to absolute temperature.
(3) Kinetic gas equation : On the basis of the above stasted postulates, the following gas equation can be derived,
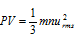
here, P = pressure exerted by the gas
V = volume of the gas
m = average mass of each molecule
n = number of molecules
u = root mean square (RMS) velocity of the gas.
(4) Calculation of kinetic energy
We know that,
K.E. of one molecule 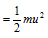
K.E. of n molecules 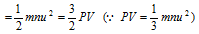
n = 1, Then K.E. of 1 mole gas 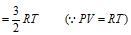
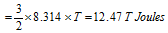
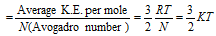
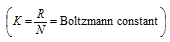
This equation shows that K.E. of translation of a gas depends only on the absolute temperature. This is usually known as the Maxwell generalization. Therefore the average kinetic energy 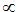 T.
T.
If T=0K (that is -273.15 degree C then, average Kinetic energy= 0. Thus, absolute zero (0K) is the temperature at which molecular motion ceases.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Kinetic theory of gases questions? Kinetic theory of gases topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Kinetic theory of gases related problems. We provide step by step Kinetic theory of gases question's answers with 100% plagiarism free content. We prepare quality content and notes for Kinetic theory of gases topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours