IUPAC Nomenclature of complex compounds
In order to name complex compounds certain rules have been framed by IUPAC. These are as stated below :
(1) Positive part of the coordination compound is named first and is followed by name of the negative part.
(2) The ligands are named first followed by the central metal. The prefixes such as di-, tri-, tetra-, and more are used to indicate the number of each type of ligand present. The prefixes bis (for the two ligands), tris (for the three ligands), and more are used when the ligands includes a number such as dipyridyl, bis (ethylenediamine).
(3) In polynuclear complexes, the bridging group is indicated in the formula of the complex by separating it from the rest of the complex by hyphens. In polynuclear complexes (a complex with two or more metal atoms), bridging ligand (this links the two metal atoms) is denoted by the prefix μ before its name.
(4) Naming of ligands : The different types of ligands that is neutral, negative or positive are named differently in a complex compound.
When a complex species has negative charge, the name of the central metal ends in - ate. For some of the elements, the ion name is based on the Latin name of the metal (for instance, argentate for silver). Some such type of latin names used (with suffix - ate) are as follows:
|
Fe
|
Ferrate
|
Cu
|
Cuperate
|
|
Ag
|
Argentate
|
Au
|
Aurate
|
|
Sn
|
Stannate
|
Pb
|
Plumbate
|
(5) Point of attachment in case unidentate ligands with more than co-ordinating atoms (ambidentate ligands) : The point of attachment in case of unidentate ligands with more than one co-ordinating atoms is either indicated by using different names for the ligands (e.g, thiocyanato and isothiocyanato) or by placing the symbol of the donor atom attached, name of the ligand is separated by a hypen.

(6) Name of the bridging groups : If a complex contains two or more central metal atoms or ions, it is termed as polynuclear. In several polynuclear complexes ligands may link the two metal atoms or ions. This type of ligands which link the two metal atoms or ions in polynuclear complexes are termed as bridge ligands. The bridge ligands are separated from rest of the complex by hyphens and denoted by the prefix μ . If there exist two or more bridging groups of the same kind, this is indicated by tri μ di- μ and more.
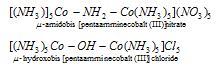
(7) If the lattice component such as water or solvent of crystallisation is present, these follow the name and are preceded by the number of these groups (molecules of solvent of crystallisation) in Arabic numerals.
For example,
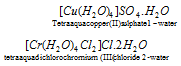
(8) The following punctuation rules should also be followed while writing name of the complex compounds.
(i) The name of the complete compound should not start a capital letter, the example of it is given as follows

(ii) The full name of complex ion should be written as one word without any gap in between.
(iii) There should be a gap between the cation and anion in case of ionic complexes.
(iv) Full name of the non-ionic complexes should be written as one word without any gap in between.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with IUPAC Nomenclature of complex compounds questions? IUPAC Nomenclature of complex compounds topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their IUPAC Nomenclature of complex compounds related problems. We provide step by step IUPAC Nomenclature of complex compounds question's answers with 100% plagiarism free content. We prepare quality content and notes for IUPAC Nomenclature of complex compounds topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours