Isothermal Expansion : In the isothermal expansion, ΔT = 0; ΔE = 0.
According to first law of thermodynamics,
ΔE = q+w thus q = -w
This shows that in the isothermal expansion, the work is done by the system at the expense of the heat absorbed.
Since for isothermal process, ΔE and ΔT are zero correspondingly, therefore, ΔH = 0
(i) Work done in the reversible isothermal expansion : Consider an ideal gas enclosed in a cylinder fitted with a weightless and frictionless piston. The cylinder near is not insulated. The external pressure, Pext is equal to pressure of the gas, Pgas.
Pext = Pgas = P
If the external pressure is decreased by an infinitesimal quantity dP, the gas will expand by the infinitesimal volume, dV. As a result of the expansion, the pressure of the gas within the cylinder falls to Pgas - dP, that is it becomes again equal to the external pressure and, thus, the piston comes to rest. This type of process is repeated for a number of times that is in each step the gas expands by a volume dV.
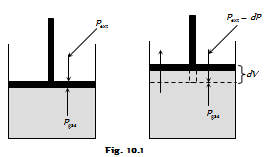
Since the system is in the thermal equilibrium with the surroundings, the infinitesimally small cooling produced because of the expansion is balanced by the absorption of the heat from the surroundings and the temperature remains constant throughout expansion.
The work done by gas in each step of the expansion can be given as follows, 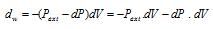
dP.dV the product of the two infinitesimal amount, can be neglected.
Therefore the total amount of work done by isothermal reversible expansion of the ideal gas from the volume V1 to volume V2 can be, given as, 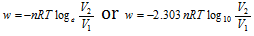
At constant temperature, according to the Boyle's law,
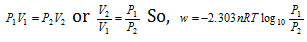
Isothermal compression work of the ideal gas might be derived similarly and it has exactly same value with positive sign.
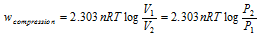
(ii) Work done in irreversible isothermal expansion : The two types of irreversible isothermal expansions are observed, which are stated as follows
(a) Free expansion
(b) Intermediate expansion.
In free expansion, external pressure is zero, which means work done is zero when the gas expands in vacuum. In the intermediate expansion, external pressure is less than the pressure of gas. So, the work done when the volume changes from V1 to V2 is can be given as
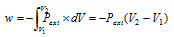
Since Pext is less than the pressure of the gas, the work done during the intermediate expansion is numerically less than work done during this reversible isothermal expansion in which the Pext is nearly equal to Pgas.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Isothermal Expansion of an Ideal Gas questions? Isothermal Expansion of an Ideal Gas topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Isothermal Expansion of an Ideal Gas related problems. We provide step by step Isothermal Expansion of an Ideal Gas question's answers with 100% plagiarism free content. We prepare quality content and notes for Isothermal Expansion of an Ideal Gas topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours