Ionic radius : It is the effective distance from the nucleus of an ion upto which it has its influence on its electron cloud.
A cation is always much smaller than the corresponding atom. Also the number of electrons removed smaller will be size of the resulting positive ion. For example, 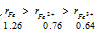 . This is due to following two factors
. This is due to following two factors
(i) A cation formed by the loss of electrons may result in the complete disappearance of the outer shell and since the remaining inner shells do not extend so far in space, the cation is a lot smaller than the metal atom. The example of it is given as follows
Sodium atom (Na) → Sodium ion (Na+)
(2, 8, 1) (2, 8)
(ii) Each time a cation is formed, ratio of the nuclear charge to the number of electrons (Z/e ratio) is increased with the result the effective nuclear charge is increased and the electrons are pulled towards the nucleus. As a result, the cation becomes smaller. The anion is always larger than the corresponding atom. For example, Atomic radius of I = 1.23 ; Ionic radii of I- = 2.16
This is again due to following two factors : (a) Since in the formation of an anion, one or more electrons are added to it, the electron cloud expands and the size of the ion increases. (b) In formation of the anion, the effective nuclear charge reduces with the result the electrons get away from nucleus and hence the anion becomes larger than the corresponding atom.
In any of the particular group, the ions of elements increase in size on moving from top to bottom.
In case of isoelectronic ions (ions having same number of electrons but different nuclear charge); the greater the nuclear charge, the greater will be the attraction for electrons and smaller will be ionic radius. Therefore size of such ions decreases. This can be given as follows
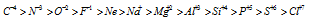

Size of ions (in decreasing order)
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Ionic radius questions? Ionic radius topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Ionic radius related problems. We provide step by step Ionic radius question's answers with 100% plagiarism free content. We prepare quality content and notes for Ionic radius topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours