Ionic radii and Radius ratio
(1) Ionic radii : The X-ray diffraction or the electron diffraction techniques gives the necessary information in context to the unit cell. From dimensions of the unit cell, it is possible to calculate the ionic radii.
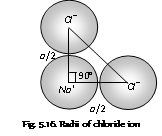
Let, cube of edge length 'a' having cations and anions say NaCl structure.
Then, rc + ra = a/2
where rc and ra are radius of cation and anion.
Radius of Cl- 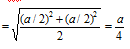
For bcc lattice say CsCl 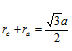
(2) Radius ratio : The ionic compounds occur in the crystalline forms. Ionic compounds are made of the cations and anions. These ions are arranged in the three dimensional array to form the aggregate of the type (A+B-)n . As, the Coulombic forces are non-directional, hence structures of such crystals are majorly governed by the ratio of the radius of cation (r+) to that of anion (r-). The ratio (r+) to (r-) (r+/r-) is called as radius ratio.
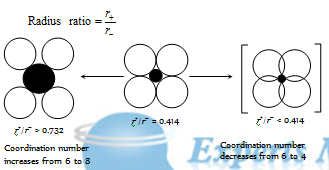
Table : Limiting Radius ratios and Structure
|
Limiting radius ratio (r+)/(r-)
|
C.N.
|
Shape
|
|
< 0.155
|
2
|
Linear
|
|
0.155 - 0.225
|
3
|
Planar triangle
|
|
0.225 - 0.414
|
4
|
Tetrahedral
|
|
0.414 - 0.732
|
6
|
Octahedral
|
|
0.732 - 0.999 or 1
|
8
|
Body-centered cubic
|
Effect of temperature and Pressure on C.N.
On applying high pressure NaCl structure having 6:6 co-ordination changes to CsCl structure having 8:8 co-ordination. Thus, increase in pressure increases the co-ordination number.
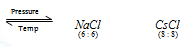
Similarly, CsCl structure on heating to about 760 K, changes to NaCl structure. In other words, increase of temperature decreases the co-ordination number.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Ionic radii and Radius ratio questions? Ionic radii and Radius ratio topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Ionic radii and Radius ratio related problems. We provide step by step Ionic radii and Radius ratio question's answers with 100% plagiarism free content. We prepare quality content and notes for Ionic radii and Radius ratio topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours