Ionic product of water
The water is a weak electrolyte and it undergoes selfionistion to a small extent.
The product of concentrations of H+ and OH- ions in water at a particular temperature is known as ionic product of water. It is selected as Kw.
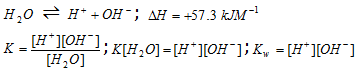
The value of Kw increases with increase of the temperature, that is the concentration H+ and OH- ions increases with increase in temperature.
The value of Kw at 25 0C is 1 * 10-14 mole/litre. As pure water is neutral in nature, ion concentration must be equal to ion concentration.
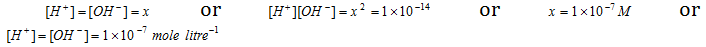
This shows that at 25 degree C, in 1 litre only 10-7 mole of water is in ionic form out of a total of about 55.5 moles.
Thus when, [H+] = [OH-]; the solution is neutral
[H+] > [OH-]; this solution is acidic in nature
[H+] < [OH-]; this solution is basic in nature
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Ionic product of water questions? Ionic product of water topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Ionic product of water related problems. We provide step by step Ionic product of water question's answers with 100% plagiarism free content. We prepare quality content and notes for Ionic product of water topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours